After bite for infants and preparation method thereof
An antipruritic technology for infants and young children, which is applied in the field of antipruritic ointment for infants and its preparation, can solve the problems of irritation and side effects, and achieve the effects of low irritation, good redness, swelling and itching, and improved utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] An antipruritic ointment for infants, characterized in that the antipruritic ointment includes A component, B component, and C component;
[0029] The A component includes the following components in weight percentage: emulsifier sorbitan olivem 1000 1.00-6.00%; cetostearyl alcohol C16 C18 0.08-3.00%, monoglyceride 0.02-2.00%, tea tree Oil 1.00-4.00%, eucalyptus oil 0.50-4.00%, clove oil 0.3-1.80%, borneol 0.20-0.50%, mint 0.60-1.80%, camphor 0.60-0.40%;
[0030] The B component includes the following components in weight percentage: Carbopol 0.08-0.40%, Xanthan Gum 0.08-0.30%, EDTA-2Na 0.01-0.08%, Water 75-85%, Cnidium Fructus Extract 1.20-3.00% %, honeysuckle extract 1.00-2.00%;
[0031] The C component includes the following components in weight percentage: 0.60-2.00% of purslane extract, 0.10-0.90% of phenoxyethanol PHG, and 0.05-0.40% of triethanolamine.
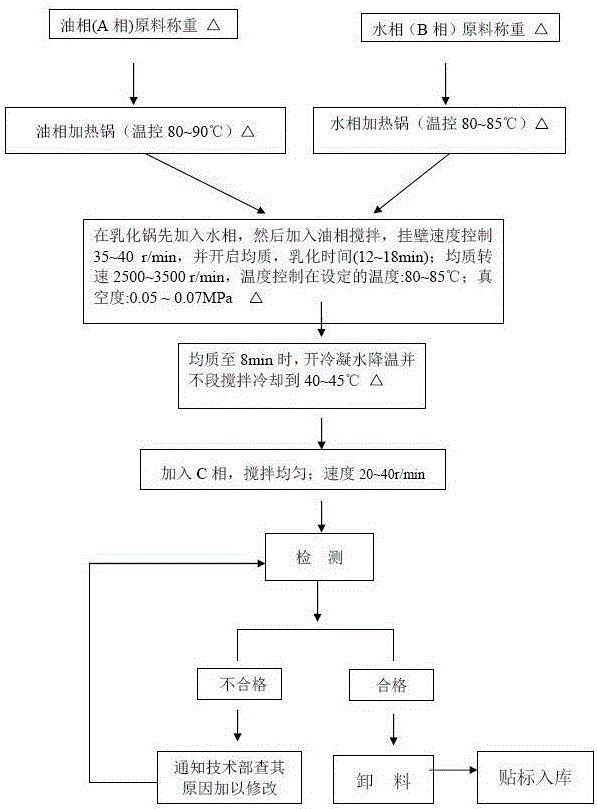
[0032] A preparation method of antipruritic ointment for infants, comprising the following steps:
[0033] ...
Embodiment 2
[0049] An antipruritic ointment for infants, comprising A component, B component and C component;
[0050] The A component includes the following components in weight percentage: emulsifier sorbitan olivem 1000 3.00-5.00%; cetostearyl alcohol C16 C18 1.00-2.60%, monoglyceride 0.06-1.40%, tea tree Oil 1.30-2.80%, eucalyptus oil 1.00-2.20%, clove oil 0.6-1.50%, borneol 0.24-0.38%, mint 0.80-1.50%, camphor 1.20-0.40%;
[0051] The B component includes the following components in weight percentage: Carbopol 0.08-0.30%, Xanthan Gum 0.12-0.26%, EDTA-2Na 0.03-0.06%, Water 77-82%, Cnidium Fructus Extract 1.60-2.40% %, honeysuckle extract 1.1-1.60%;
[0052] The C component includes the following components in weight percentage: 0.80-1.60% of purslane extract, 0.40-0.70% of phenoxyethanol PHG, and 0.10-0.30% of triethanolamine.
[0053] The preparation method is the same as in Example 1.
Embodiment 3
[0055] An antipruritic ointment for infants, comprising A component, B component and C component;
[0056] The A component includes the following components in weight percentage: emulsifier sorbitan olivem 1000 4.00%; cetostearyl alcohol C16 C18 2.00%, monoglyceride 1.00%, tea tree oil 2.00%, eucalyptus Oil 1.50%, clove oil 1.10%, borneol 0.30%, mint 1.00%, camphor 0.25%;
[0057] The B component includes the following components in weight percentage: Carbopol 0.25%, Xanthan Gum 0.20%, EDTA-2Na 0.05%, Water 79.35%, Cnidium Fructus Extract 2.00%, Honeysuckle Extract 1.20%;
[0058] The C component includes the following components in weight percentage: 1.00% of purslane extract, 0.60% of phenoxyethanol PHG, and 0.20% of triethanolamine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com