Neuraminidase inhibitor and preparation method thereof
A technology for neuraminidase and inhibitors, which is applied in the field of neuraminidase inhibitors and their preparation, can solve the problems of low yield of neuraminidase inhibitors, achieve economic benefits, enhance electrophilicity, and avoid Effect of overprotonation of amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
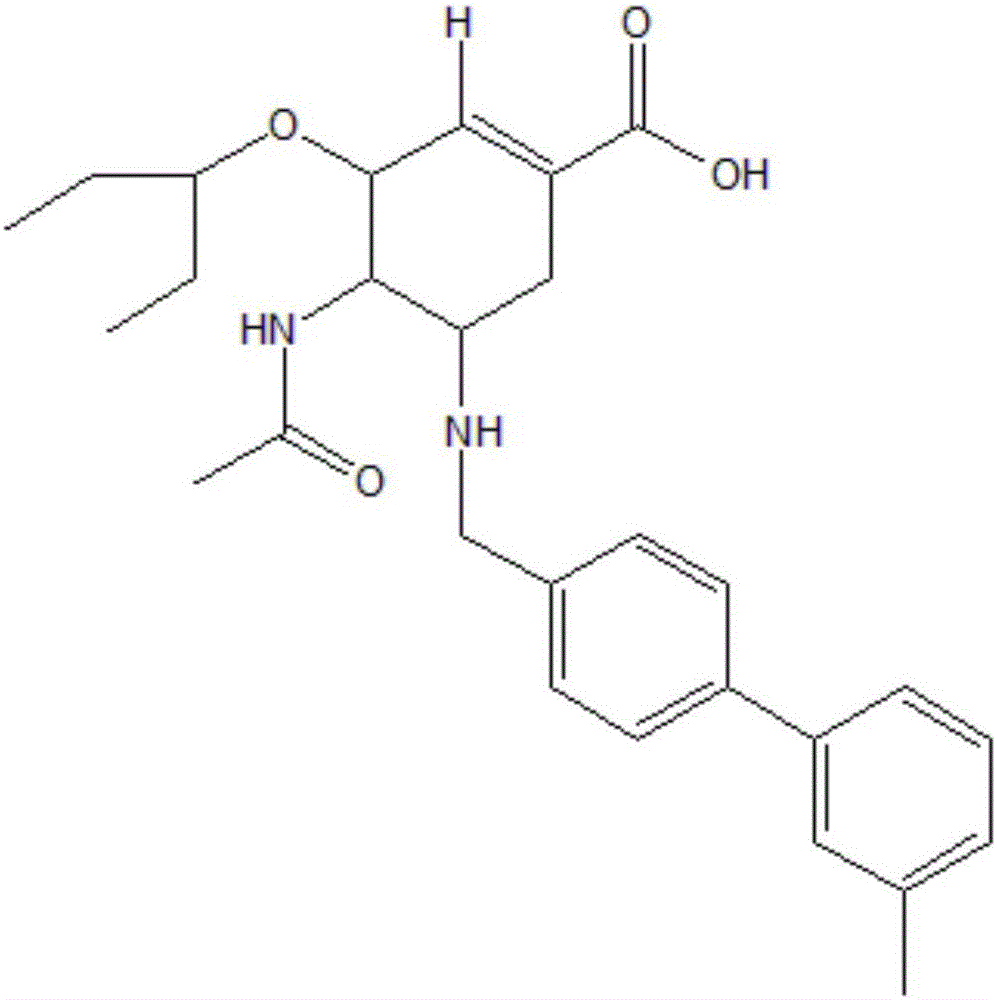
[0022] (1) First, mix 0.5582g of raw material oseltamivir phosphate and 0.3203g of biphenylaldehyde into 25ml of ethanol solution, add 0.012g of anhydrous acetic acid as a catalyst and react at 25°C for 15 hours; then slowly add 0.1229g Sodium cyanoborohydride was solid, reacted at 25°C for 15 hours, the reaction was complete as monitored by TLC, and the intermediate was obtained by post-experimental treatment.
[0023] (2) Accurately weigh 0.4643g of the intermediate compound and add it to a mixed solvent of methanol and deionized water (v / v, 3:1), add 0.1179g of solid sodium hydroxide, and react at 50°C for 6 hours. After the reaction was completed, the pH of the solution was adjusted to weak acidity with anhydrous acetic acid, the plate was monitored by TLC, and the final product was obtained by column chromatography separation with a final yield of 58.7%.
[0024] For the target compound C 28 h 36 N 2 o 4 Carry out high-resolution mass spectrometry and nuclear magnetic...
Embodiment 2
[0026] Same as Example 1, in the first step reaction solution of the synthetic intermediate, slowly add 0.012g of anhydrous acetic acid as a catalyst, and increase the reaction time of the synthetic intermediate to 20 hours, and increase the reaction time of the synthetic final product to 8 hours hours, the product yield was 31.7%.
Embodiment 3
[0028] Same as in Example 1, slowly add 0.012 g of anhydrous acetic acid as a catalyst to the first step reaction liquid for synthesizing the intermediate, raise the reaction temperature of the synthetic intermediate compound to 30°C, and raise the reaction temperature of the synthetic final product to 55°C °C, the yield of the final product was 36.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com