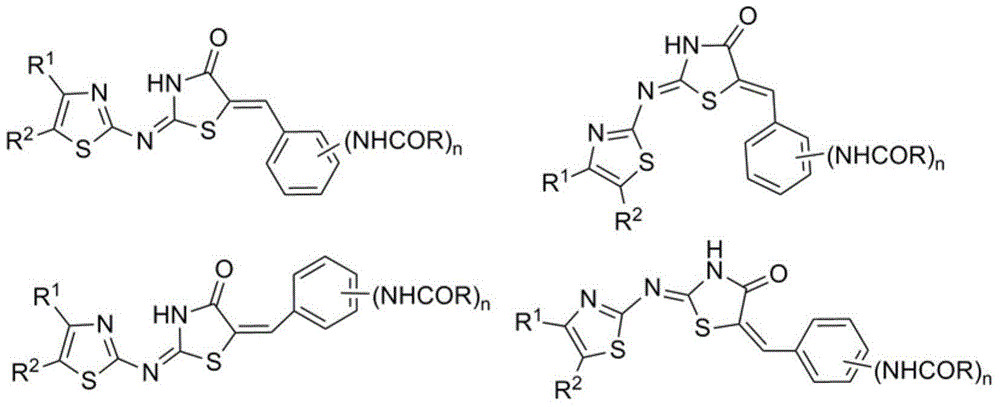
2-(thiazol-2-yl)imino-5-(benzylidene)thiazolidin-4-one, and preparing method and applications thereof
A technology of benzylidenethiazoline and iminothiazoline is applied in the application of influenza virus neuraminidase inhibitor, and the preparation field of 2-imino-5-benzylidenethiazolin-4-one
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 2-[(4-tert-butylthiazol-2-yl)imino]thiazolin-4-one (2)
[0030]
[0031] 4.36g (25mmol) 4-tert-butylthiazol-2-amine, 30mL dichloromethane, stir to dissolve, add 3.45g (25mmol) anhydrous potassium carbonate, stir at room temperature for 30min, drop 2ml (25mmol) chloroacetyl chloride, Reaction 1.5h. The reaction solution was poured into ice water, extracted with dichloromethane, washed with saturated aqueous sodium carbonate, combined organic phases, dried over anhydrous sodium sulfate, precipitated, and recrystallized from ethanol to obtain 5.50 g of white solid 1, yield 87.7%, m.p.113-115 ℃.
[0032] Dissolve 2.30g (9mmol) of compound 1, 1.31g (13.5mmol) of potassium thiocyanate in 30ml of ethanol, and heat to reflux for 2.5h. After cooling, a solid was precipitated, filtered with suction, and dried to obtain 2.10 g of yellow solid 2, yield 90.0%, m.p.200-202°C. 1 HNMR (CDCl 3 , 400MHz) δ: 1.33(s, 9H, 3×CH 3 ), 3.88 (s, 2H, CH 2 ), 6.64 (s, 1H, t...
Embodiment 2
[0034] Preparation of (2E,5E)-2-(4-tert-butylthiazol-2-yl)imino-5-(3-nitrobenzylidene)thiazolin-4-one
[0035]
[0036] 35ml of acetic acid was dissolved in 0.65g of sodium acetate to prepare a buffer solution with pH=4~5, 2.0mmol of intermediate 2 and 4.0mmol of 3-nitrobenzaldehyde were added, and the reaction was heated under reflux for 10h. Cool, wash with water, filter, and recrystallize from ethanol to give yellow solid (2E,5E)-2-(4-tert-butylthiazol-2-yl)imino-5-(3-nitrobenzylidene)thiazoline-4 - Ketone, yield 66%, m.p.225-226°C. 1 HNMR (CDCl 3 , 400MHz) δ: 1.40(s, 9H, 3×CH 3 ), 6.73 (s, 1H, thiazole ring), 7.69 (t, J=8.0Hz, 1H, C 6 h 4 ), 7.86 (d, J=8.4Hz, 2H, =CH), 8.28 (d, J=8.0Hz, 1H, C 6 h 4 ), 8.49 (s, 1H, C 6 h 4 ).
Embodiment 3
[0038] Preparation of (2E,5E)-2-(4-tert-butylthiazol-2-yl)imino-5-(4-nitrobenzylidene)thiazolin-4-one
[0039]
[0040] The operation method is the same as in Example 2, compound 2 and 4-nitrobenzaldehyde react for 10 h to obtain yellow solid (2E, 5E)-2-(4-tert-butylthiazol-2-yl)imino-5-(4 -Nitrobenzylidene)thiazolin-4-one, yield 77%, m.p.270~272°C. 1 H NMR (CDCl 3 , 400MHz) δ: 1.38(s, 9H, 3×CH 3 ), 6.73 (s, 1H, thiazole ring), 7.72 (d, J=8.9Hz, 2H, C 6 h 4 ), 7.84 (s, 1H, = CH), 8.35 (d, J = 8.6Hz, 2H, C 6 h 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com