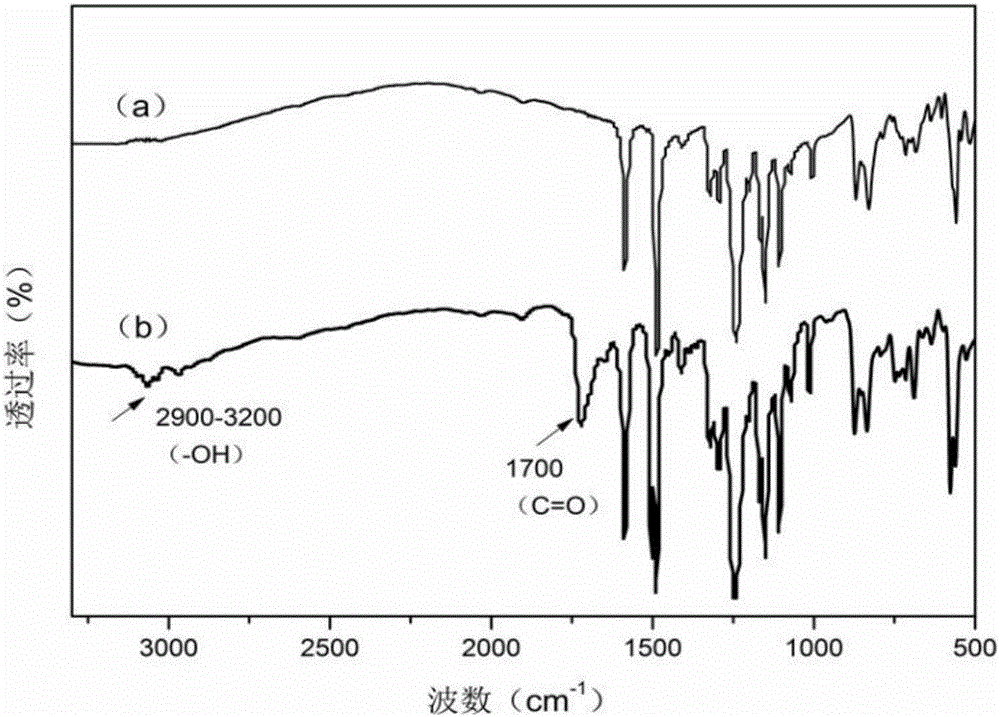
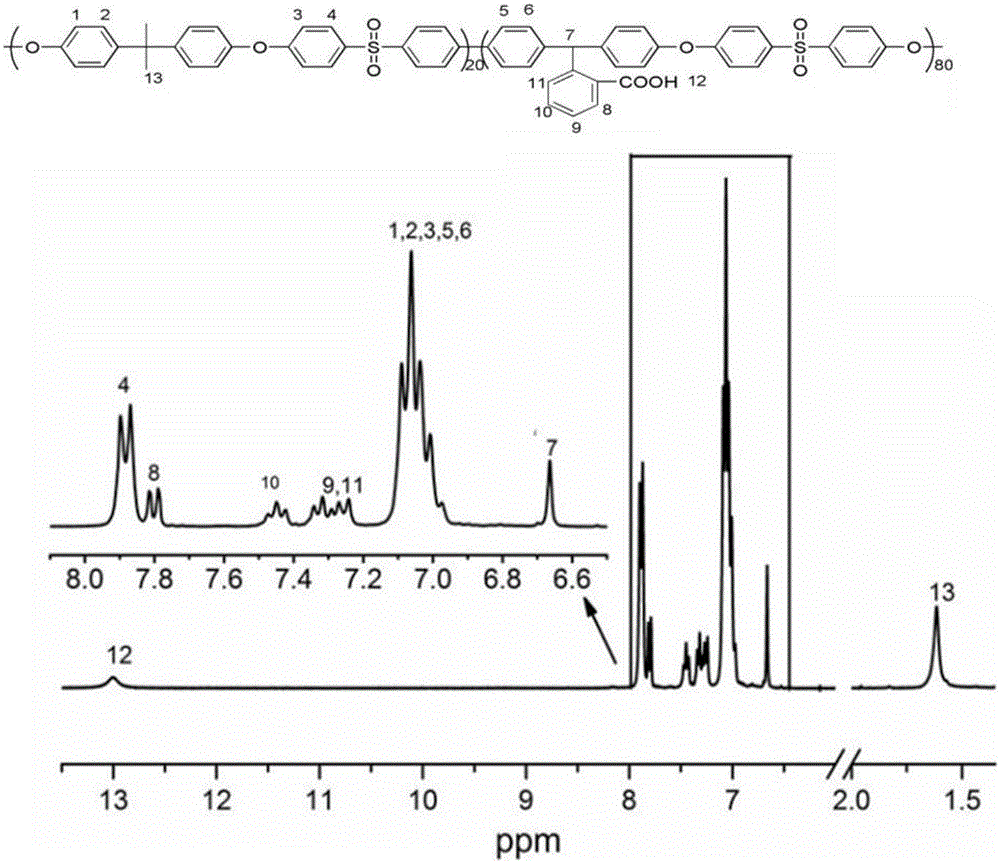
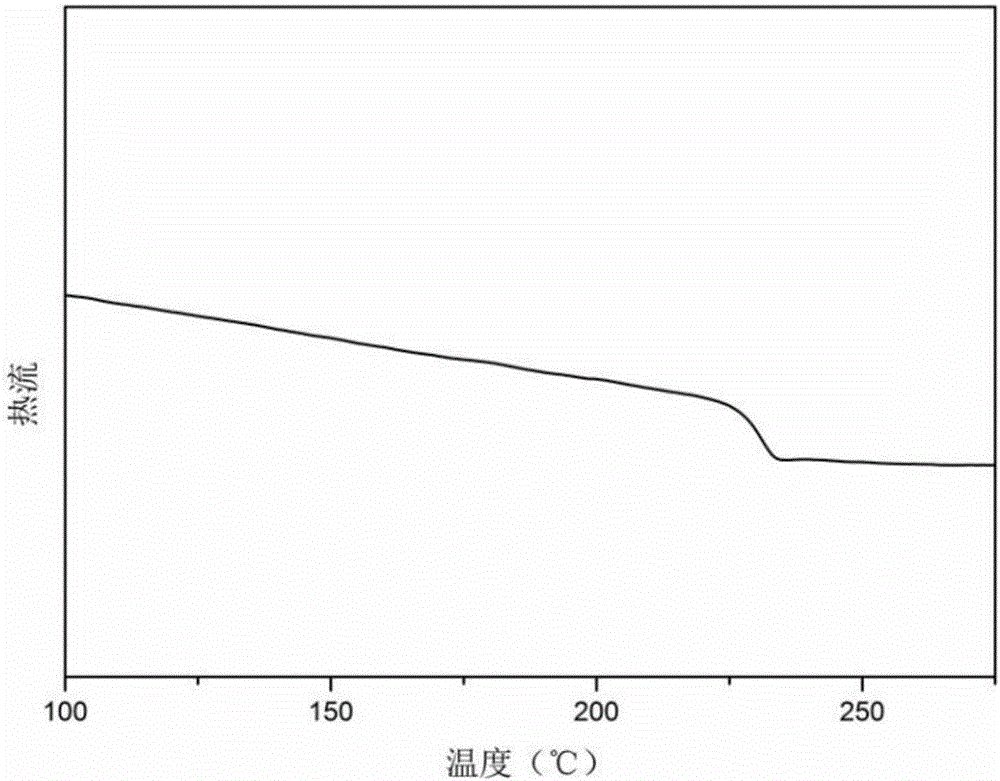
Carboxyl-side-group-containing polyarylether copolymers and preparation method thereof, and application of copolymers in aspect of ultrafiltration membrane hydrophilic modification
A kind of technology like copolymer and polyarylene ether, which is applied in the field of ultrafiltration membrane preparation, can solve the problems of easy pollution of hydrophobicity, and achieve the effects of enhanced hydrophilicity, cheap raw materials and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: This embodiment is a blank pure PSF homopolymer.
[0039] Add 150mmol 4,4-bis(4-hydroxyphenyl)propane (bisphenol A, BPA), 150mmol 4,4′-bis Chlorodiphenyl sulfone (DDS) and 300mmol anhydrous potassium carbonate, 200.3946g dimethyl sulfoxide (DMSO) as the reaction solvent (solid content: 25%), 50mL toluene as the water carrier. Under the protection of nitrogen, slowly heat the reaction system to 150°C, keep the reflux state, use toluene to take out the water generated in the reaction in the form of azeotropy, distill off the excess toluene after 3 hours, and then slowly raise the temperature to 160°C React and polymerize for 3 hours to obtain a polymer solution with high viscosity. Discharge the polymer solution into deionized water, and then pulverize it with a pulverizer, and wash it with boiling water several times to remove residual solvents and inorganic salts. Boil and wash with ethanol four times to remove unreacted monomers, and dry in a vacuum oven...
Embodiment 2
[0040] Embodiment 2: This embodiment prepares polysulfone copolymer containing carboxyl side group
[0041] Add 30mmol 4,4′-dihydroxytriphenylmethane-2-carboxylic acid (phenolphthalein, PPL), 120mmol 4,4- Bis(4-hydroxyphenyl)propane (bisphenol A, BPA), 150mmol 4,4'-dichlorodiphenyl sulfone (DDS) and 300mmol anhydrous potassium carbonate, with 207.3870g dimethyl sulfoxide (DMSO) as Reaction solvent (solid content is 25%), 50mL toluene is used as water-carrying agent. Under the protection of nitrogen, slowly heat the reaction system to 150°C, keep the reflux state, use toluene to take out the water generated in the reaction in the form of azeotropy, distill off the excess toluene after 3 hours, and then slowly raise the temperature to 160°C React and polymerize for 3 hours to obtain a polymer solution with a higher viscosity, which is discharged in deionized water. After being pulverized by a pulverizer, deionized water is boiled and washed several times to remove residual solv...
Embodiment 3
[0042] Example 3: This example prepares a polysulfone copolymer containing carboxyl side groups.
[0043] Add 60mmol 4,4′-dihydroxytriphenylmethane-2-carboxylic acid (phenolphthalein, PPL), 90mmol 4,4- Bis(4-hydroxyphenyl)propane (bisphenol A, BPA), 150mmol 4,4'-dichlorodiphenyl sulfone (DDS) and 300mmol anhydrous potassium carbonate, with 215.6715g dimethyl sulfoxide (DMSO) as Reaction solvent (solid content is 25%), 50mL toluene is used as water-carrying agent. Under the protection of nitrogen, slowly heat the reaction system to 150°C, keep the reflux state, use toluene to take out the water generated in the reaction in the form of azeotropy, distill off the excess toluene after 4 hours, and then slowly raise the temperature to 160°C React and polymerize for 4 hours to obtain a polymer solution with high viscosity, which is discharged into deionized water. After being pulverized by a pulverizer, deionized water is boiled and washed several times to remove residual solvents ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com