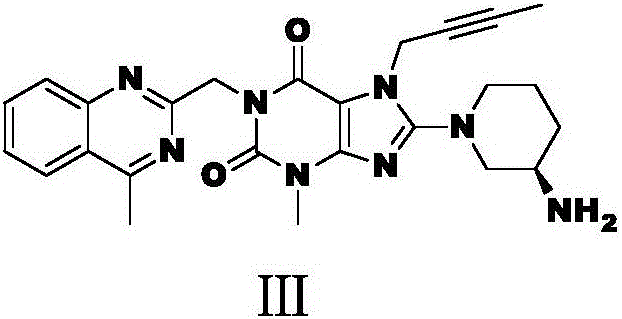
Industrial production method of linagliptin
A production method and intermediate technology, applied in the field of industrial production of linagliptin, can solve the problems of low purity of intermediates, large environmental pollution, complicated operation, etc., and achieve good impurity removal effect, good repeatability, and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
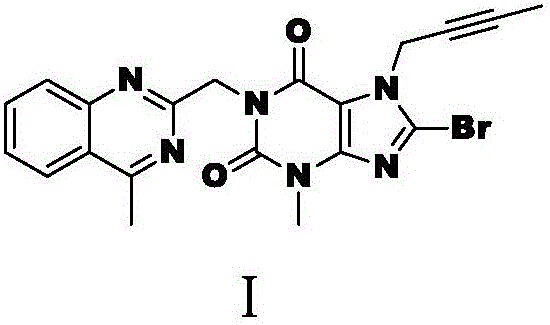
[0017] 1. Preparation of Intermediate I:
[0018] Add dipolar aprotic organic solvent N-methylpyrrolidone 32kg, anhydrous potassium carbonate 4kg, 8-bromo-7-(2-butyne)-3-methylxanthine 2.8kg, 2-chloromethyl-4- Methyl-quinazoline 4kg, potassium iodide 0.78kg, stir, heat up to 75°C and insulate and stir for 3hr, detect 8-bromo-7-(2-butyne)-3-methylxanthine raw material residue is no more than 1.0% stop, Cool down to below 40°C, add 48kg of purified water dropwise at a constant speed under stirring, continue to cool down and cool to 15°C, keep stirring for 2 hours, centrifuge, and rinse the solid with 15kg of purified water to obtain a khaki-yellow wet product, which is placed in a double-cone dryer for 50 ℃ and dried under reduced pressure (-0.09Mpa) for about 18hr until the weight loss on drying was no more than 1.0%, and 5.5kg of intermediate I was obtained as a khaki-yellow dry product, with a purity of 98.8% and a yield of 90%. The structural formula of intermediate I was as...
Embodiment 2
[0027] 1. Preparation of Intermediate I:
[0028] Add dipolar aprotic organic solvent N-methylpyrrolidone 29kg, anhydrous potassium carbonate 3.6kg, 8-bromo-7-(2-butyne)-3-methylxanthine 2.8kg, 2-chloromethyl-4 -Methyl-quinazoline 3.2kg, potassium iodide 0.66kg, stir, heat up to 44°C and stir for 3hr, detect 8-bromo-7-(2-butyne)-3-methylxanthine raw material residues are not more than 1.0% Stop, cool down to below 40°C, add 42kg of purified water dropwise at a constant speed under stirring, continue to cool down and cool to 15°C, keep stirring for 2 hours, centrifuge, and rinse the solid with 13kg of purified water to obtain a khaki-yellow wet product, put it in a double-cone dryer Dry at 50°C under reduced pressure (-0.09Mpa) for about 18hr until the weight loss on drying does not exceed 1.0%, and 4.35kg of intermediate I is obtained as a khaki-yellow dry product.
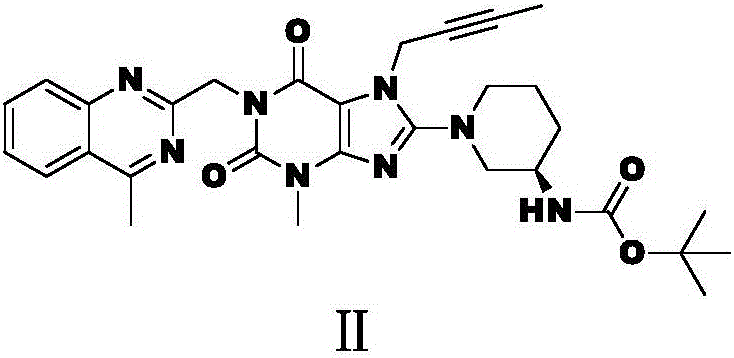
[0029] 2. Preparation of intermediate II:
[0030] Add 40kg of dipolar aprotic organic solvent N,N-dimethylfo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com