Diabetes treatment pharmaceutical composite and preparation method thereof
A composition and drug technology, applied in the field of epalrestat tablets and their preparation, can solve the problems of large differences in dissolution rate between tablets, low average dissolution rate of epalrestat tablets, etc. The effect of increasing the average dissolution rate, increasing the content and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
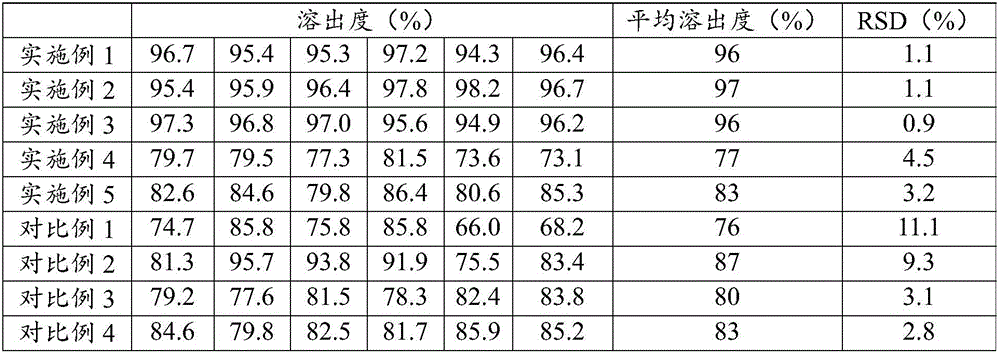
Embodiment 1
[0053] The present embodiment epalrestat tablet, its raw material consists of:
[0054] Epalrestat 50mg, Mannitol 50mg, Hydroxypropylcellulose 5mg, Poloxamer 1883mg, Cross-linked carboxymethyl calcium 6mg, Magnesium stearate 1mg, Appropriate amount of water, Appropriate amount of 50% ethanol aqueous solution, Hypromellose Base cellulose 9mg, titanium dioxide 5.3mg, polyethylene glycol 0.7mg;
[0055] Its preparation method comprises the following steps:
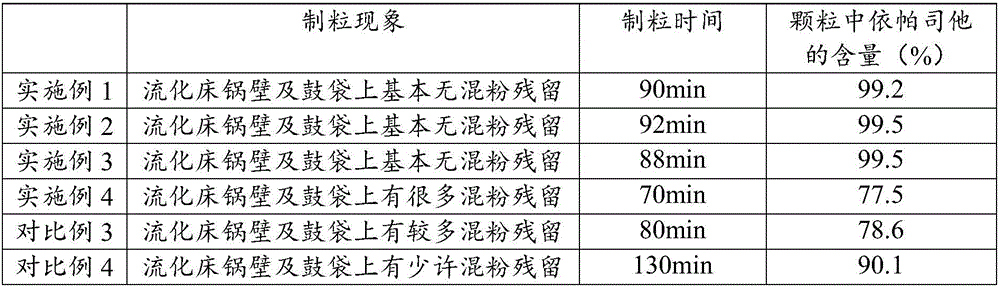
[0056] Take the raw and auxiliary materials of the prescription amount respectively, pulverize and sieve respectively, and the particle diameter (D90) of epalrestat is 15 μm;
[0057] Add the prescribed amount of hydroxypropyl cellulose and the prescribed amount of poloxamer 188 to an appropriate amount of water, and stir until dissolved to obtain a solution;
[0058] The epalrestat of the prescribed amount and the mannitol of the prescribed amount are granulated by the fluidized bed granulation method with the solution, dr...
Embodiment 2
[0062] The present embodiment epalrestat tablet, its raw material consists of:
[0063] Epalrestat 50mg, Mannitol 44mg, Hydroxypropylcellulose 5mg, Poloxamer 1889mg, Cross-linked carboxymethylcalcium 6mg, Magnesium stearate 1mg, Appropriate amount of water, Appropriate amount of 50% ethanol aqueous solution, Hypromellose Base cellulose 9mg, titanium dioxide 5.3mg, polyethylene glycol 0.7mg;
[0064] Its preparation method comprises the following steps:
[0065] Take the raw and auxiliary materials of the prescription amount respectively, pulverize and sieve respectively, and the particle diameter (D90) of epalrestat is 15 μ m;
[0066] Add the prescribed amount of hydroxypropyl cellulose and the prescribed amount of poloxamer 188 to an appropriate amount of water, and stir until dissolved to obtain a solution;
[0067] The epalrestat of the prescribed amount and the mannitol of the prescribed amount are granulated by the fluidized bed granulation method with the solution, dr...
Embodiment 3
[0071] The present embodiment epalrestat tablet, its raw material consists of:
[0072] Epalrestat 50mg, Mannitol 48mg, Hydroxypropylcellulose 5mg, Poloxamer 1885mg, Cross-linked carboxymethylcalcium 6mg, Magnesium stearate 1mg, Appropriate amount of water, Appropriate amount of 50% ethanol aqueous solution, Hypromellose Base cellulose 9mg, titanium dioxide 5.3mg, polyethylene glycol 0.7mg;
[0073] Its preparation method comprises the following steps:
[0074] Take the raw and auxiliary materials of the prescription amount respectively, pulverize and sieve respectively, and the particle diameter (D90) of epalrestat is 15 μ m;
[0075] Add the prescribed amount of hydroxypropyl cellulose and the prescribed amount of poloxamer 188 to an appropriate amount of water, and stir until dissolved to obtain a solution;
[0076] The epalrestat of the prescribed amount and the mannitol of the prescribed amount are granulated by fluidized bed granulation in the solution, dried, and gran...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com