Method for determining content of silver, copper, lead and zinc elements in gold concentrate
A technology of element content and gold concentrate, applied in the field of analytical chemistry, can solve the problems of consumables, time-consuming, labor-intensive, etc., and achieve the effect of simplifying the experimental process, expanding the measurement range, and improving the accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
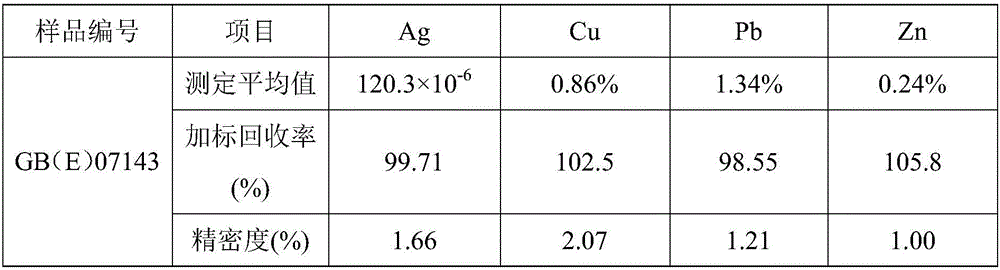
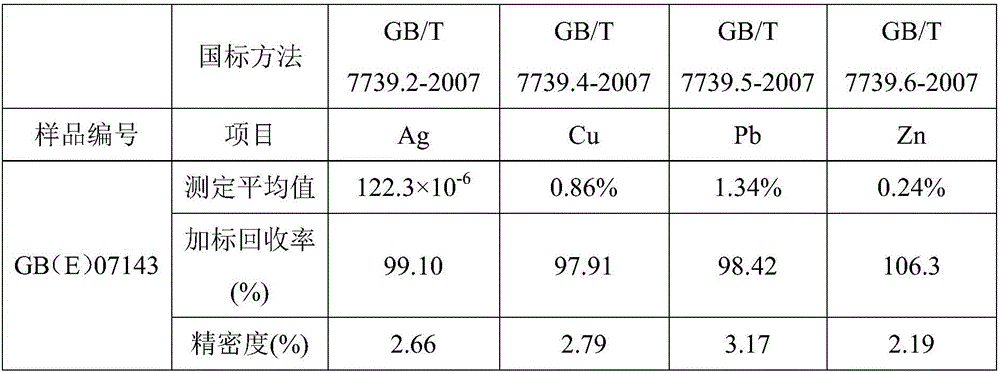
[0027] After crushing the sample, the particle size is 0.038-0.074mm. Dry the sample at 105°C for 1 hour, weigh 0.4992g of the sample, place the sample in a 250mL beaker, add a small amount of water to wet it, add 15mL of hydrochloric acid, and cover it with a watch glass. , heat at 100°C for 5 minutes, remove and cool slightly, then add 8 mL of nitric acid, dissolve for 10 minutes, add 3 mL of perchloric acid, continue heating until thick white smoke comes out, steam until wet salt, remove and cool, add 10 mL of hydrochloric acid, Rinse the cup wall and watch glass with water, heat to dissolve the salt, dilute the solution to a 100mL volumetric flask, dilute to the mark with pure water, mix well, and let it stand still for clarification. Along with the sample blank, it was determined by atomic absorption spectrometer.
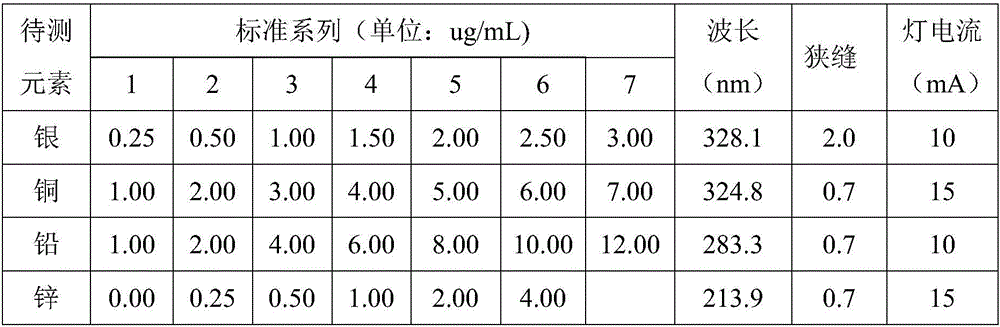
[0028] Preparation of silver, copper, lead and zinc standard series with different gradients, see Table 1; constant volume solution to directly measure silver...
Embodiment 2
[0036] After crushing the sample, the particle size is 0.038-0.074mm. Dry the sample at 105°C for 1 hour, weigh 0.3525g of the sample, place the sample in a 250mL beaker, add a small amount of water to wet it, add 18mL of hydrochloric acid, and cover it with a watch glass. , heated at 100°C for 8 minutes, removed and cooled slightly, then added 9 mL of nitric acid, dissolved for 15 minutes, added 4 mL of perchloric acid, continued to heat until thick white smoke appeared, steamed to wet salt, removed and cooled, added 13 mL of hydrochloric acid, Rinse the cup wall and watch glass with water, heat to dissolve the salt, dilute the solution to a 100mL volumetric flask, dilute to the mark with pure water, mix well, and let it stand still for clarification. Along with the sample blank, it was determined by atomic absorption spectrometer.
[0037] Prepare silver, copper, lead and zinc standard series with different gradients, see Table 1; constant volume solution to directly measure...
Embodiment 3
[0043] After crushing the sample, the particle size is 0.038-0.074mm. Dry the sample at 105°C for 1 hour, weigh 0.2008g of the sample, place the sample in a 250mL beaker, add a small amount of water to wet it, add 20mL of hydrochloric acid, and cover it with a watch glass. , heat at 100°C for 10 minutes, remove and cool slightly, then add 10mL of nitric acid, dissolve for 20 minutes, add 5mL of hydrofluoric acid, add 5mL of perchloric acid, continue heating until thick white smoke comes out, steam until wet salt, remove Cool down, add 15mL hydrochloric acid, rinse the cup wall and watch glass with water, heat to dissolve the salt, dilute the solution to a 100mL volumetric flask, dilute to the mark with pure water, mix well, stand still and clarify, bring a sample blank with it, and use atomic absorption spectrometer to measure.
[0044] Prepare silver, copper, lead and zinc standard series with different gradients, see Table 1; constant volume solution directly measures the si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com