Osteoarthritis diagnosing product and application thereof
A technology for osteoarthritis, products, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 High-throughput sequencing screening of differentially expressed genes
[0073] 1. Sampling
[0074] From October 2012 to December 2015, patients with osteoarthritis who were treated in the Department of Orthopedics of Peking Union Medical College Hospital were collected. A total of 10 cases were collected as the case group. A total of 4 cases were collected from patients with other diseases who were hospitalized in the Department of Orthopedics during the same period as controls. The synovial fluid samples of all subjects were obtained, numbered and stored in a -80°C low-temperature refrigerator.
[0075] The patients in the case group were all in line with the diagnostic criteria of knee OA and underwent knee arthroplasty; the control group were patients with meniscus injury and cruciate ligament injury who underwent arthroscopic surgery. According to the 1995 American Rheumatology Association diagnostic criteria for osteoarthritis, a patient with severe os...
Embodiment 2
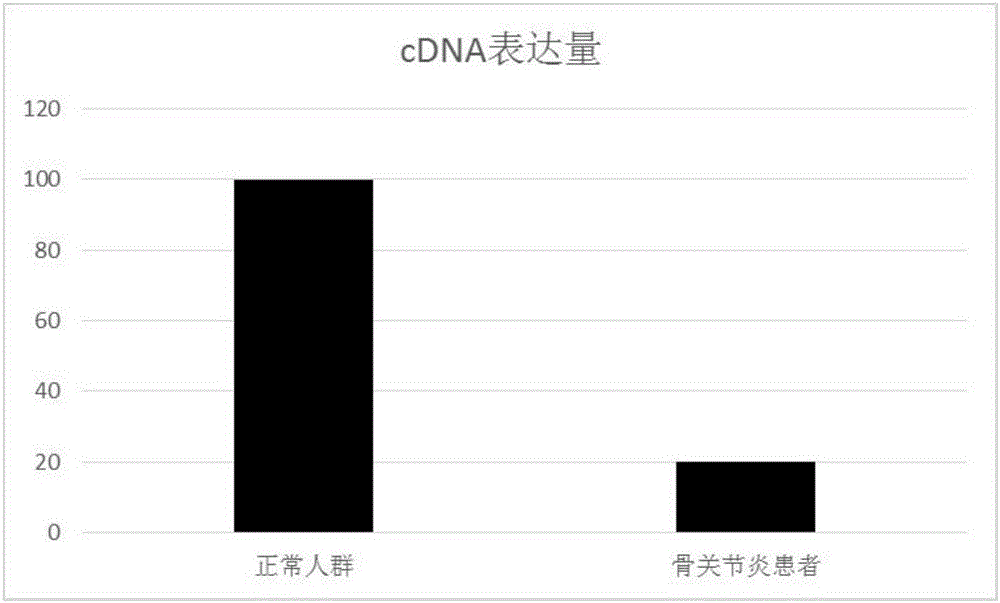
[0097] Example 2 RT-PCR verification of CRNN gene expression in osteoarthritis patients and control synovial fluid
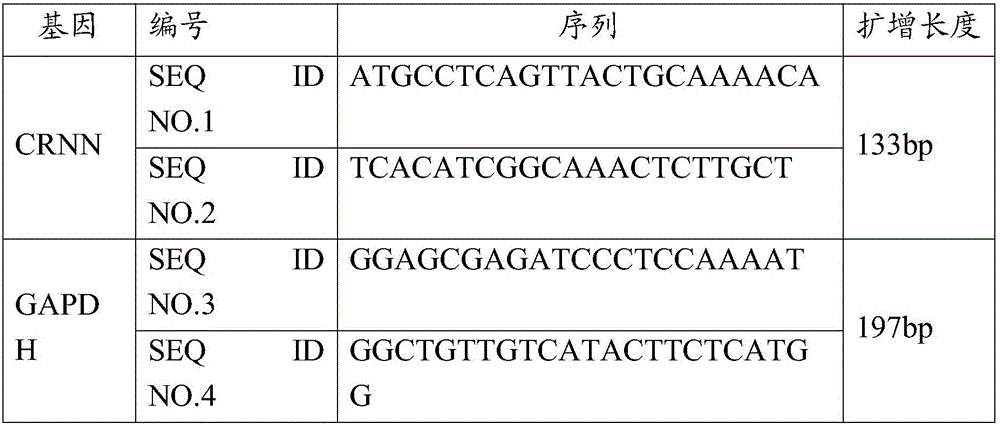
[0098] 1. Materials
[0099] The cartilage tissues of 42 cases of osteoarthritis patients and 8 cases of control cartilage tissues were selected and grouped and numbered. The patients in the case group were all in line with the diagnostic criteria of knee OA and underwent knee arthroplasty; the control group were patients with meniscus injury and cruciate ligament injury who underwent arthroscopic surgery.
[0100] 2. Method
[0101] 2.1 Carry out total RNA extraction to synovial fluid, with the extraction method of embodiment 1.
[0102] 2.2 Synthesis of cDNA by reverse transcription
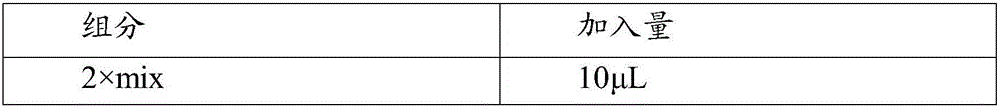
[0103] use III Reverse Transcriptase (invitrogen, Cat. No. 18080-044) was used for cDNA reverse transcription, and the experimental operation was carried out according to the product manual. The specific operation was as follows:
[0104] Using a reverse transcription kit, ...
Embodiment 3
[0123] Example 3 Gene chip verification of CRNN gene expression in synovial fluid of patients with osteoarthritis and controls
[0124] 1, the acquisition of material, with embodiment 2.
[0125] 2, the extraction of total RNA, with the method for embodiment 1.
[0126] 3. Gene chip verification
[0127] After the total RNA was linearized and amplified, the cy3-UTP labeled and fluorescently labeled cRNAs were purified using the RNEASY Mini Kit, and the labeled cRNAs were fragmented with Amhion’s RNA Fragmentation Reagents. Human whole gene expression profile chips (4x 44K genes) from Agilent Company of the United States were used, hybridized in a chip hybridization oven at 65°C for 17 hours, then eluted, stained, and finally scanned with an Agilent DNA MicroarrayScanner scanner.
[0128] After the hybridized chip reads the data points by the chip scanner, the data is imported into the analysis software, and the genes whose absolute value of the natural logarithm of the ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com