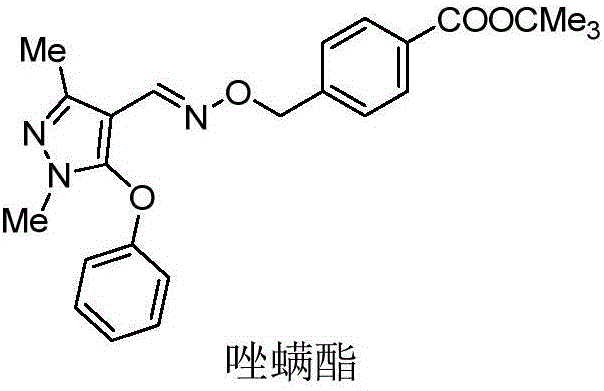
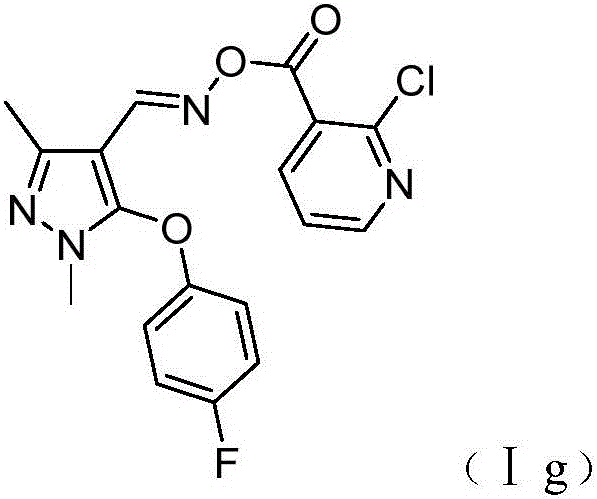
Preparation and application of pyrazole oxime ester compound containing 2-chloropyridine structure
A technology of ester compound and pyrazole oxime is applied in the field of pesticides to achieve the effect of excellent control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
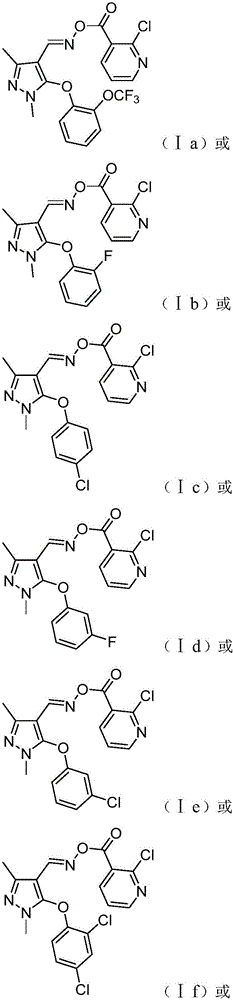
[0027] In a 100mL four-necked flask, add 4mmol II a, 3mL pyridine and 20mL dichloromethane. Under stirring at room temperature, 5 mmol of III in dichloromethane solution (10 mL) was slowly added dropwise thereto. After dropping, continue to stir at room temperature for 12 hours. The solvent was distilled off under reduced pressure, 60 mL of dichloromethane was added to the residue, washed with saturated brine, dried over anhydrous magnesium sulfate, precipitated, separated and purified by column chromatography to obtain the target compound Ia with a yield of 51%; 1 H NMR (400MHz, CDCl 3 ): δ8.53(d, J=4.0Hz, 1H, Py-H), 8.09~8.11(s, 2H, CH=N and Py-H), 7.40(d, J=8.0Hz, 1H, Ar- H),7.32~7.36(m,1H,Py-H),7.25~7.29(m,1H,Ar-H),7.18~7.21(m,1H,Ar-H),6.80(d,J=8.0Hz ,1H,Ar-H),3.65(s,3H,N-CH 3 ),2.52(s,3H,CH 3 ).
Embodiment 2
[0029]
[0030] In a 100mL four-necked flask, add 3mmol II b, 5mL triethylamine and 25mL dichloromethane. Under stirring at room temperature, 4.2 mmol of III in dichloromethane solution (15 mL) was slowly added dropwise thereto. After dropping, continue to stir at room temperature for 16 hours. The solvent was distilled off under reduced pressure, 60 mL of dichloromethane was added to the residue, washed with saturated brine, dried over anhydrous magnesium sulfate, precipitated, separated and purified by column chromatography to obtain the target compound Ib with a yield of 53%; 1H NMR (400MHz , CDCl 3 ): δ8.53(d, J=4.8Hz, 1H, Py-H), 8.14(s, 1H, CH=N), 8.10(d, J=7.6Hz, 1H, Py-H), 7.33~7.36 (m,1H,Py-H),7.08~7.25(m,3H,Ar-H),6.87~6.91(m,1H,Ar-H),3.69(s,3H,N-CH 3 ),2.49(s,3H,CH 3 ).
Embodiment 3
[0032]
[0033] In a 100mL four-neck flask, add 5mmol II c, 10mmol sodium bicarbonate and 20mL acetonitrile. Under stirring at room temperature, 6 mmol of III in acetonitrile solution (15 mL) was slowly added dropwise thereto. After dropping, the reaction was heated to reflux for 14 hours. The solvent was distilled off under reduced pressure, 50 mL of dichloromethane was added to the residue, washed with saturated brine, dried over anhydrous magnesium sulfate, precipitated, separated and purified by column chromatography to obtain the target compound Ic with a yield of 63%; 1 H NMR (400MHz, CDCl 3 ): δ8.52(d, J=4.0Hz, 1H, Py-H), 8.14(s, 1H, CH=N), 8.09(d, J=8.0Hz, 1H, Py-H), 7.30~7.35 (m,3H,Py-H and Ar-H),6.99(d,J=8.0Hz,2H,Ar-H),3.63(s,3H,N-CH 3 ),2.49(s,3H,CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com