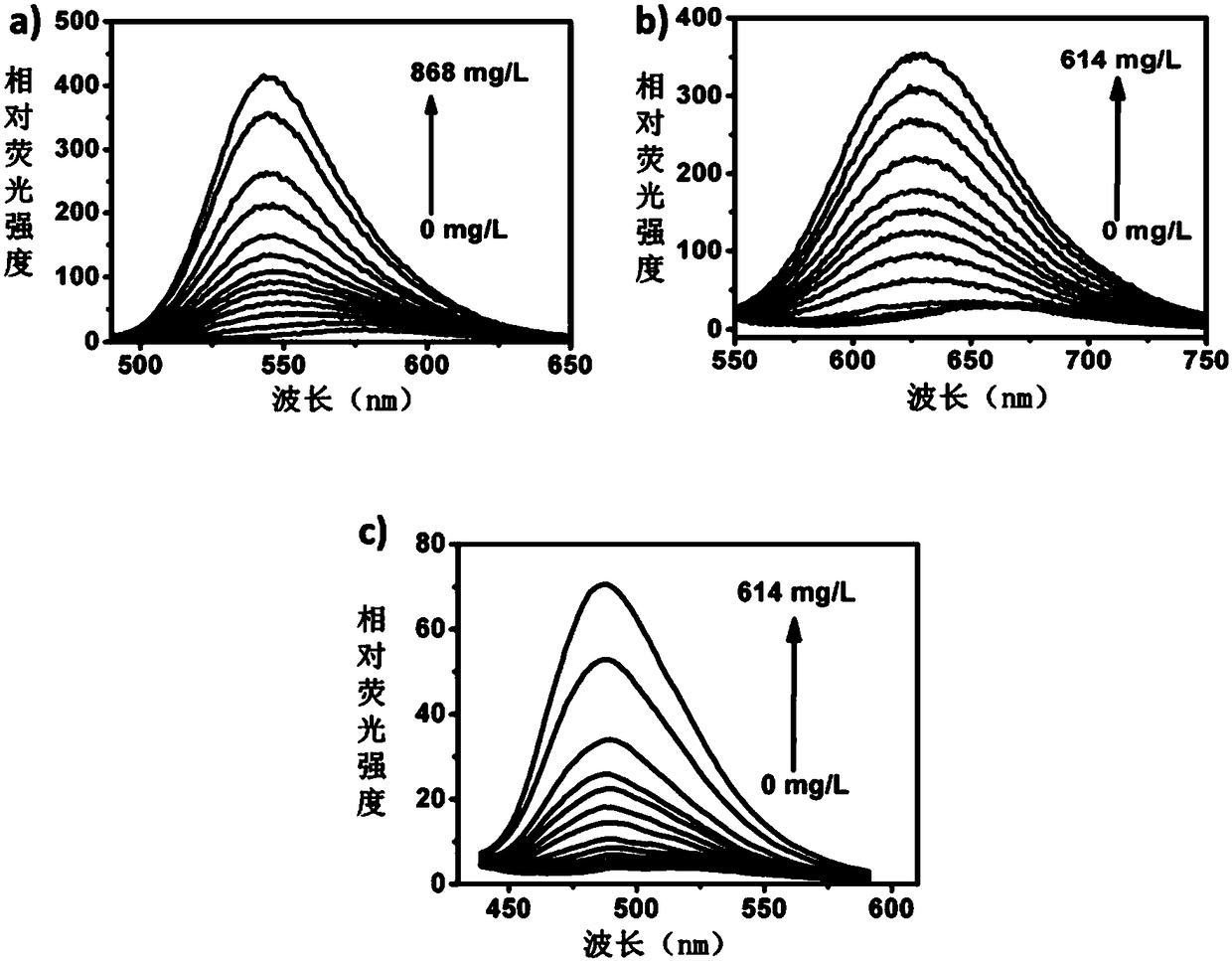
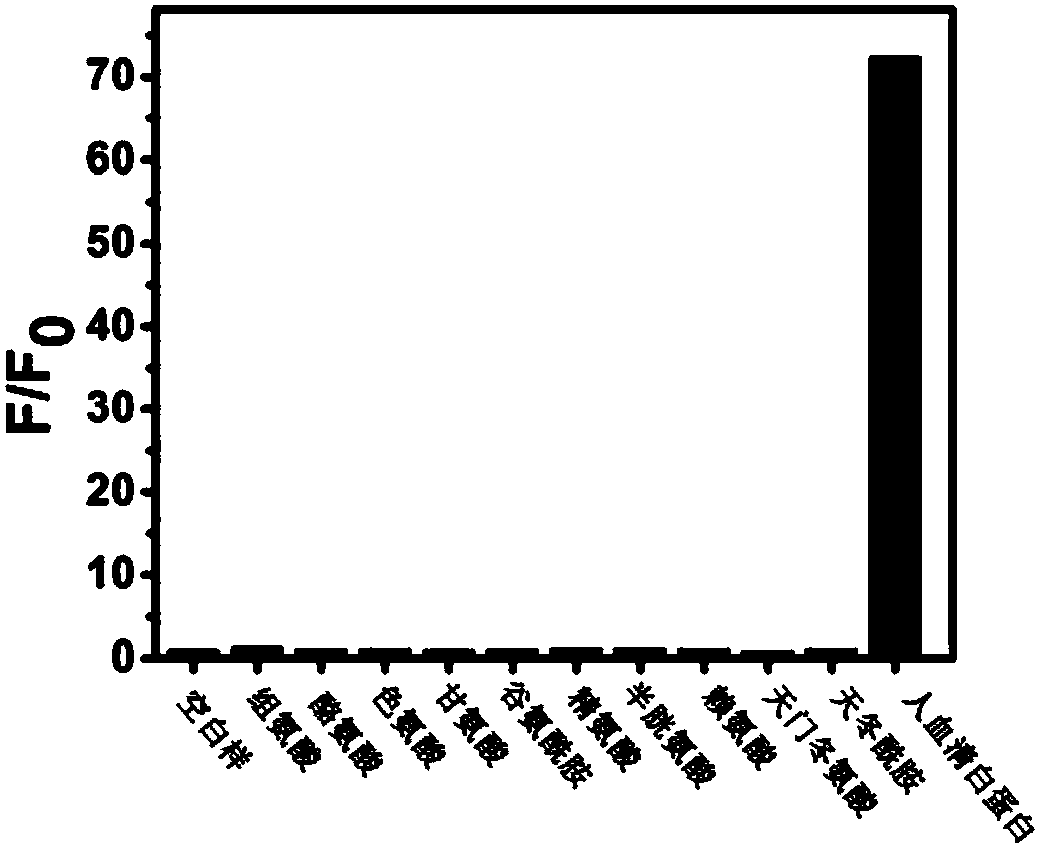
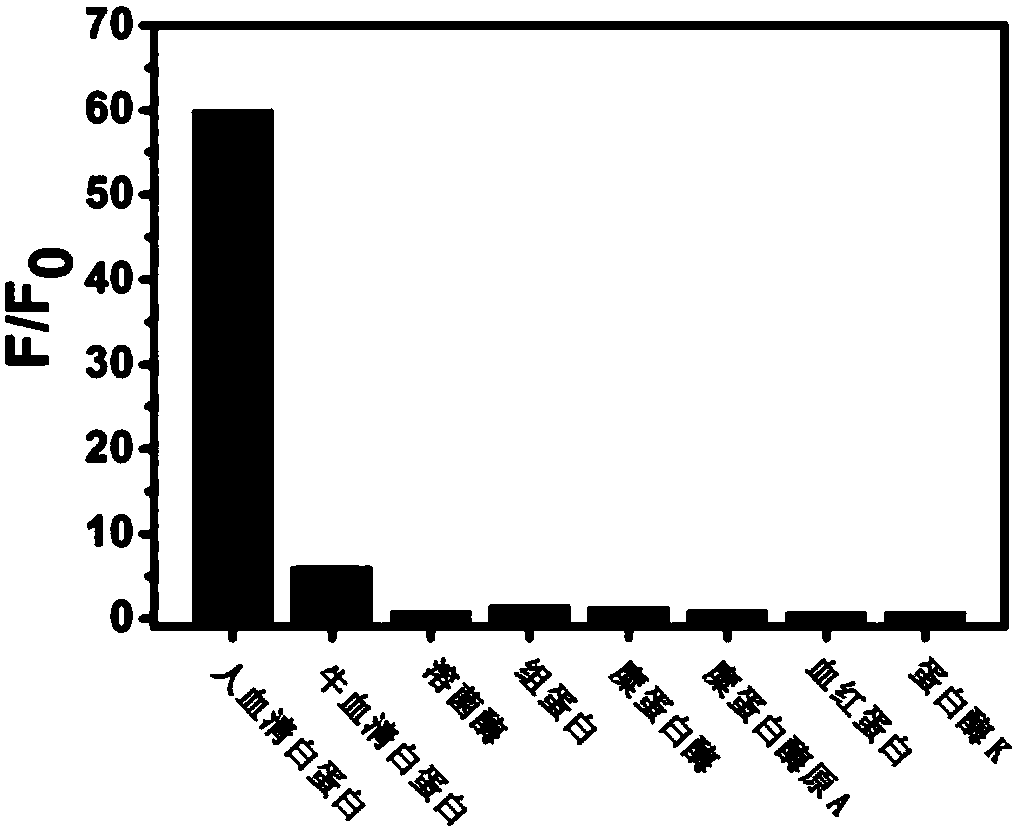
A Class of Small Molecule Fluorescent Probes and Their Applications
A fluorescent probe and small molecule technology, applied in the direction of fluorescence/phosphorescence, analytical materials, luminescent materials, etc., to achieve good selectivity, good selectivity, and good biological application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of fluorescent probe compound ES1:
[0059]
[0060] Add p-dimethylaminobenzaldehyde (1.49g, 10mmol) and rhodanine (1.33g, 10mmol) into a round bottom flask filled with 10mL of toluene, then add 200mg of ammonium acetate and 1mL of glacial acetic acid as catalysts, and heat up to 115°C Reflux reaction. After reacting for 24 hours, the solvent was evaporated under reduced pressure, and the red solid ES1 (78.25%) was obtained by column separation. 1 H NMR (500MHz, DMSO-D6), δ: 3.04(s, 6H), 4.27(s, 1H), 6.82(t, J=10Hz, 2H), 7.42(t, J=10Hz, 2H), 7.53( s,1H); 13 CNMR(500MHz,DMSO-D6),δ:112.172,117.313,119.757,132.872,133.253,151.759,169.371,176.645,194.973,105.280ppm; TOF MS:m / zcalcd for[M+H] + :265.0469,found:265.0458.
Embodiment 2
[0062] Synthesis of fluorescent probe compound ES2:
[0063]
[0064] Add 4-dimethylaminocinnamaldehyde (1.75g, 10mmol) and rhodanine (1.33g, 10mmol) into a round-bottomed flask with 12mL of toluene, then add 200mg of ammonium acetate and 1mL of glacial acetic acid as catalysts, and heat up to 115 ℃ reflux reaction. After reacting for 24 hours, the solvent was evaporated under reduced pressure and separated by chromatography to obtain dark brown solid ES2 (65.46%). 1 H NMR (500MHz, DMSO-D6), δ: 2.99(s, 6H), 3.10(q, J=25Hz, 1H), 6.72(d, J=5Hz, 3H), 7.21(q, J=45Hz, 2H ),7.51(d,J=10Hz,2H); 13 C NMR(500MHz,DMSO-D6),δ:45.75,111.89,112.10,118.48,123.31,129.41,129.73,130.97,145.41,151.45ppm; TOFMS:m / z calcd for[M+H] + :291.0626,found:291.0618.
Embodiment 3
[0066] Synthesis of fluorescent probe compound ES3:
[0067]
[0068] Add p-dimethylaminobenzaldehyde (1.49g, 10mmol) and 2,4-thiazolidinedione (1.17g, 10mmol) into a round-bottomed flask filled with 10mL of toluene, then add 200mg of ammonium acetate and 1mL of glacial acetic acid as Catalyst, heated to 115 ° C reflux reaction. After reacting for 24 hours, the solvent was evaporated under reduced pressure, and the yellow solid ES3 (85.60%) was obtained by column separation. 1 H NMR (500MHz, DMSO-D6), δ: 3.01(s, 6H), 6.83(d, J=10Hz,
[0069] 2H), 7.42(d, J=5Hz, 2H), 7.67(s, 1H), 12.31(s, 1H); 13 C NMR (500MHz, DMSO-D6), δ: 110.79, 110.91, 111.95, 120.02, 115.63, 119.79, 132.10, 132.91, 133.82, 139.29, 151.44,
[0070] 167.48,168.11ppm; TOF MS:m / z calcd for[M-H] - :247.0541,found:247.0545.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com