Thienopyrrole quinone compound, preparation method and semiconductor device comprising the material
A compound and thiophene technology, applied in the field of thienopyrrole quinone compound, preparation method and semiconductor equipment containing the material, can solve the problems of low bipolar field-effect mobility and limited quantity, and achieve low LUMO energy level , Strong self-assembly ability, good planarity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
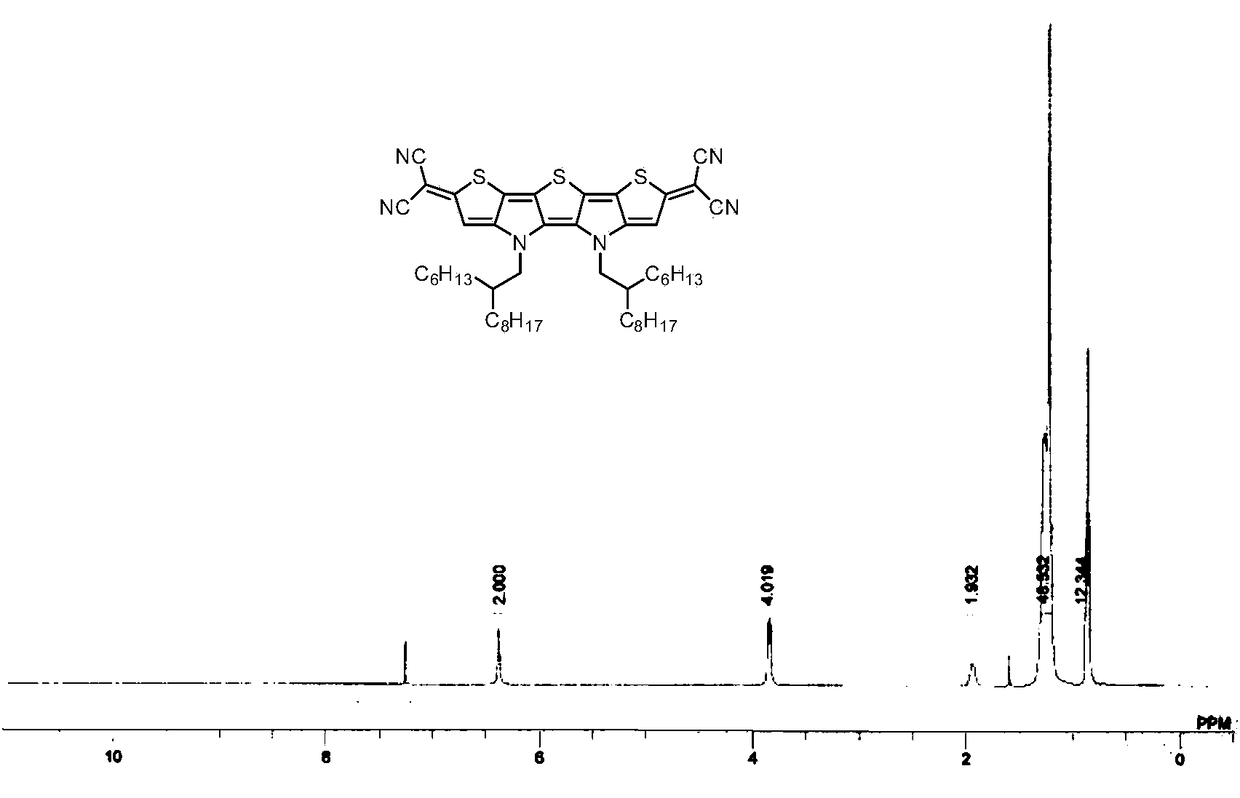
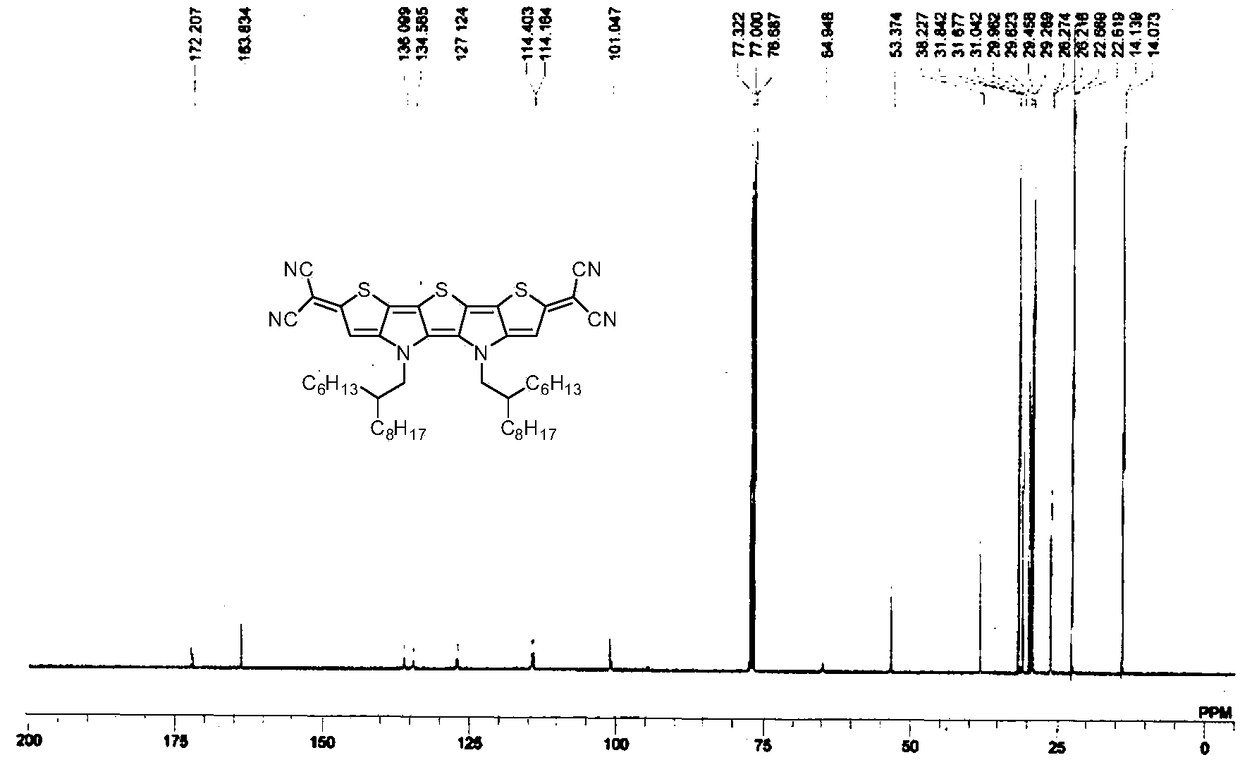
[0037] Embodiment 1: the synthesis of compound Ia
[0038]
[0039] At 0°C, under nitrogen protection, malononitrile (46.2 mg, 0.7mmol), after the foam disappeared, it was raised to room temperature and reacted for 30 minutes. The prepared malononitrile anion solution was transferred via cannula to a solution containing compound IIa (136.5 mg, 0.14 mmol), tetrakis(triphenylphosphine) palladium (32.4 mg, 0.028 mmol) and 1,2-dimethoxy In a 50 mL three-neck flask containing ethyl ethane (10 mL), the reaction was heated under reflux for 3 hours under the protection of nitrogen. Then the reaction temperature was lowered to room temperature, and exposed to the air, dilute hydrochloric acid (10mL, 1M) was added, stirred in an ice-water bath for 30 minutes, extracted with ether (30mL×3), the combined organic phases were washed with saturated brine, and Dry over anhydrous magnesium sulfate, remove the organic solvent by rotary evaporation, and the residue is separated by silica ge...
Embodiment 2
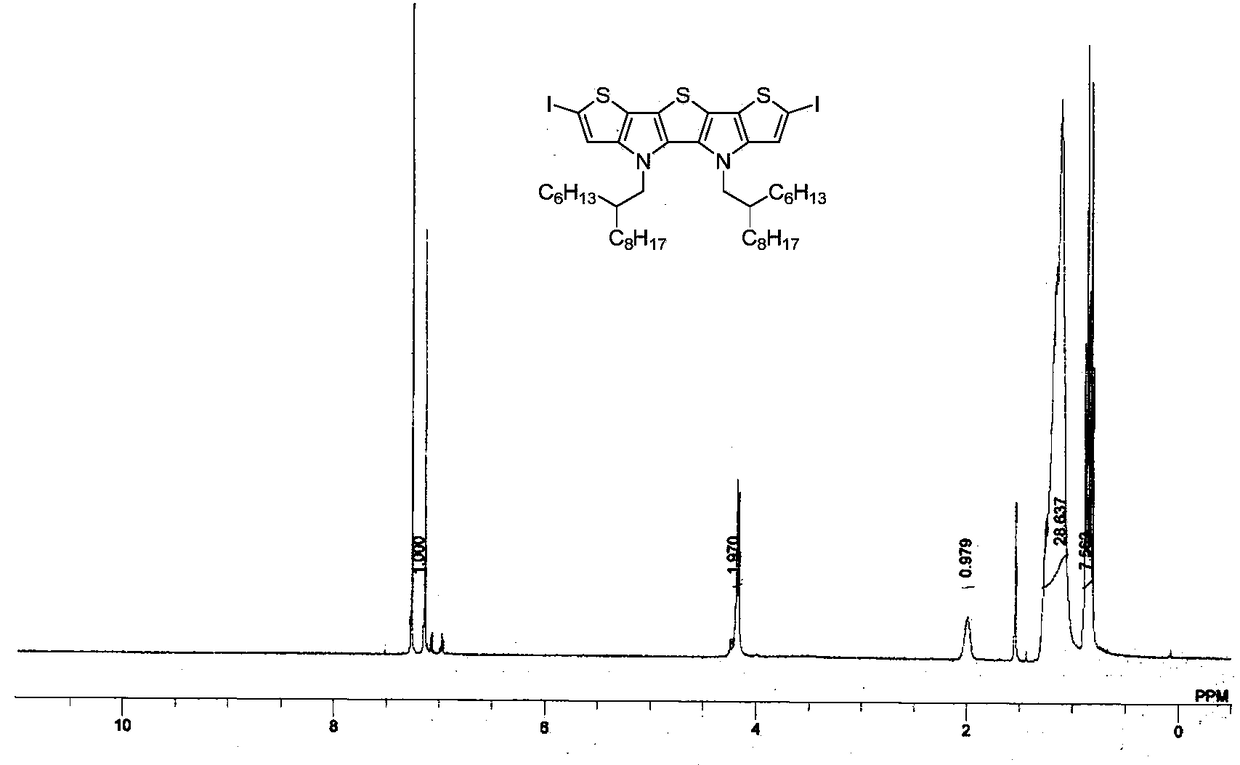
[0040] Embodiment 2: the synthesis of compound IIa
[0041]
[0042] Under nitrogen protection at -78°C, n-butyllithium (1.6M in hexane, 248 μL, 0.396mmol) was slowly added dropwise into a 10mL three-necked flask containing compound IV (130.2mg, 0.18mmol) and tetrahydrofuran (2mL), Keep stirring at low temperature for 30 minutes, add elemental iodine (100.5mg, 0.396mmol), warm to room temperature, continue stirring for 2 hours, add saturated sodium thiosulfate solution (10mL) to quench, and extract with ether (30mL×3), The organic phases were combined and washed with saturated brine, dried over anhydrous magnesium sulfate, and the solvent was removed by rotary evaporation to obtain the crude compound IIa (yellow oil, 173.8 mg, yield: 99%). 1 H NMR (400MHz, CDCl 3 )δ7.14(s,2H),4.17(d,J=8.0Hz,4H),1.99(m,2H),1.26–1.12(m,48H),0.89–0.80(m,12H); 13 C NMR (100MHz, CDCl 3 )δ144.0, 129.9, 121.2, 120.8, 116.3, 68.8, 53.5, 39.0, 31.9, 31.7, 31.0, 29.9, 29.6, 29.4, 29.3, 26.13, 26.0...
Embodiment 3
[0043] Embodiment 3: the synthesis of compound IV
[0044]
[0045] Add compound V (282.0mg, 0.5mmol), sodium tert-butoxide (768.8mg, 8.0mmol), bis(dibenzylideneacetone) palladium (28.8mg, 0.05mmol), 1,1' - Bis(diphenylphosphino)ferrocene (110.9mg, 0.2mmol) and toluene (10mL), stirred at 25°C for 20 minutes, then added compound VI (280.1mg, 1.16mmol), heated to 110°C for 10 hours . After cooling to room temperature, water (20 mL) was added, extracted with ether (30 mL×3), the combined organic phases were washed with saturated brine, dried over anhydrous magnesium sulfate, the organic solvent was removed by rotary evaporation, and the residue was separated by silica gel column chromatography ( Eluent: n-hexane) to obtain compound IV (white solid, 181.0 mg, yield: 50%). 1 H NMR (400MHz, CDCl 3 )δ7.06(d, J=4.8Hz, 2H), 6.98(d, J=5.6Hz, 2H), 4.26(d, J=8.0Hz, 4H), 2.06(m, 2H), 1.25–1.11( m,48H),0.89–0.81(m,12H); 13 C NMR (100MHz, CDCl 3 )δ144.4,130.6,121.5,116.6,116.1,111.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com