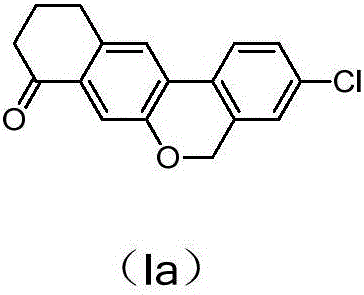
Preparation method of benzochromene derivative
A compound and mixture technology, applied in the field of preparation of benzochromene derivatives, can solve the problems of difficulty in adapting to large-scale industrial production, high price, high production cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation of embodiment 1 compound 2
[0094]
[0095] Under the protection of nitrogen, 14 kg (22.9 mol) of the compound and 20 L of THF were sequentially added into a 50 L reactor, and the temperature was lowered to -70°C. Control the temperature below -70°C and add 6.8 kg of n-BuLi dropwise, and then stir for 0.5 hours below -70°C after the drop. Control the temperature below -70°C and add 4.75kg of triisopropyl borate dropwise. Pour it into 20L (1N) dilute hydrochloric acid, separate the layers, and extract the aqueous phase with EA10L once more. The organic phases were combined, washed once with 10 L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 22.4 kg of compound with a yield of 85%.
Embodiment 2
[0096] The preparation of embodiment 2 compound 4
[0097] method 1:
[0098]
[0099] Under the protection of nitrogen, 3500g of compound, 3L of THF, 2382g of compound, 626g of potassium carbonate, 3L of deionized water, and 6g of tetrakis(triphenylphosphopalladium) were successively added into a 10L four-necked flask, and the temperature was raised to reflux for overnight reaction. TLC monitors that the reaction is complete (about 16 hours), and then pours into 3L of ice water, separates the liquid, and extracts the aqueous phase once with EA1L. The organic phases were combined, washed once with 2 L of saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 4482 g of compound with a yield of 96%.
[0100] HNMR (CDCl 3 ,400MHz):δ7.62(s,1H),7.49-7.11(m,6H),4.69(1H,s),4.50(2H,s).
[0101] Method 2:
[0102]
[0103] Add compound 350g, THF300mL, compound 238.2g, potassium carbonate 62.6g, deionized water 300mL, Pd(dppf)Cl 2 0.6g, heat...
Embodiment 3
[0105] The preparation of embodiment 3 compound 5
[0106] method 1:
[0107]
[0108] Under nitrogen protection, 450 g of the compound, 500 mL of DMSO, and 35.6 g of potassium tert-butoxide were sequentially added into a 1 L four-necked flask, and the temperature was raised to 60° C. for 1 hour. TLC monitors the disappearance of raw materials, pours into 1L of ice water, and extracts with MTBE1Lx3. The organic phases were combined, washed once with 1 L of water and once with 1 L of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 37.5 g of compound with a yield of 82%, HPLC=90%.
[0109] Method 2:
[0110]
[0111] Under nitrogen protection, 450 g of compound, 500 mL of DMF, K 2 CO 3 43.7g, heated up to 60°C and reacted for 1 hour. TLC monitors the disappearance of raw materials, pours into 1L of ice water, and extracts with MTBE1Lx3. The organic phases were combined, washed once with 1 L of water and once with 1 L of satu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com