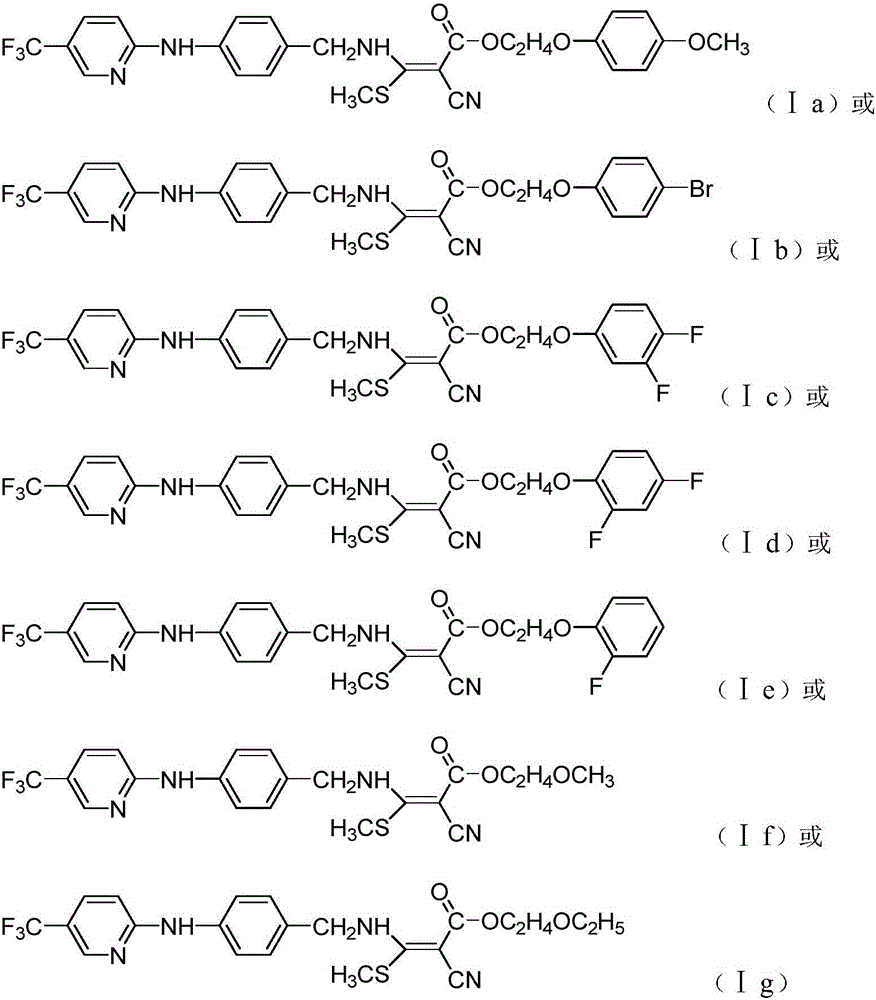
Preparation and application of cyanoacrylate compound with pyridine diarylamine structures
A technology of cyanoacrylate and pyridine diarylamine, which is applied in the field of pesticides, can solve problems such as environmental pollution, and achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
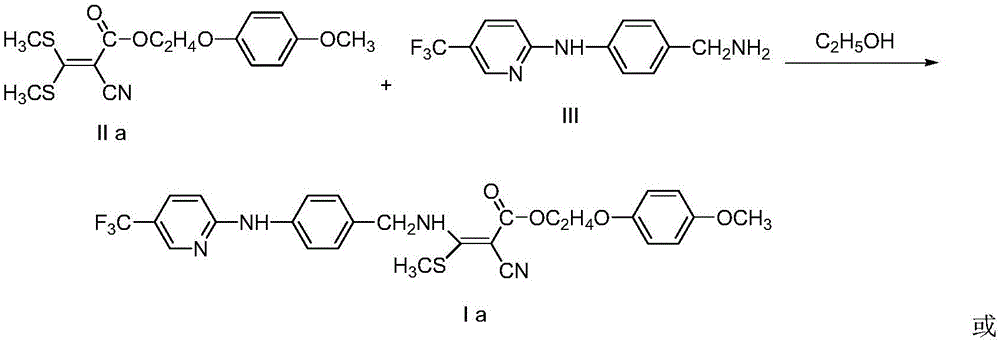
Embodiment 1
[0026]
[0027] In a 50mL round bottom flask, add 5mmolIIa, 4mmolIII and 20mL absolute ethanol, heat to reflux for 8 hours, evaporate the solvent to dryness, add 30mL water to the residue, extract with 30mL ethyl acetate, combine the organic layers, wash with water, anhydrous Dried over magnesium sulfate, filtered with suction, evaporated the solvent under reduced pressure, separated and purified by column chromatography to obtain the target compound Ia with a yield of 61%; 1 HNMR (400MHz, d 6 -DMSO): δ10.20(s,1H,NH),9.67(s,1H,NH),8.49(s,1H,Py-H),7.87(dd,J 1 =2.4Hz,J 2 =2.0Hz,1H,Py-H),7.71(d,J=8.4Hz,2H,Ar-H),7.27(d,J=8.4Hz,2H,Ar-H),6.83-6.96(m,5H ,Ar-HandPy-H),4.75-4.76(m,2H,CH 2 ),4.40(t,J=4.4Hz,2H,CH 2 ), 4.14(t, J=4.4Hz, 2H, CH 2 ),3.69(s,3H,OCH 3 ),2.62(s,3H,SCH 3 ).
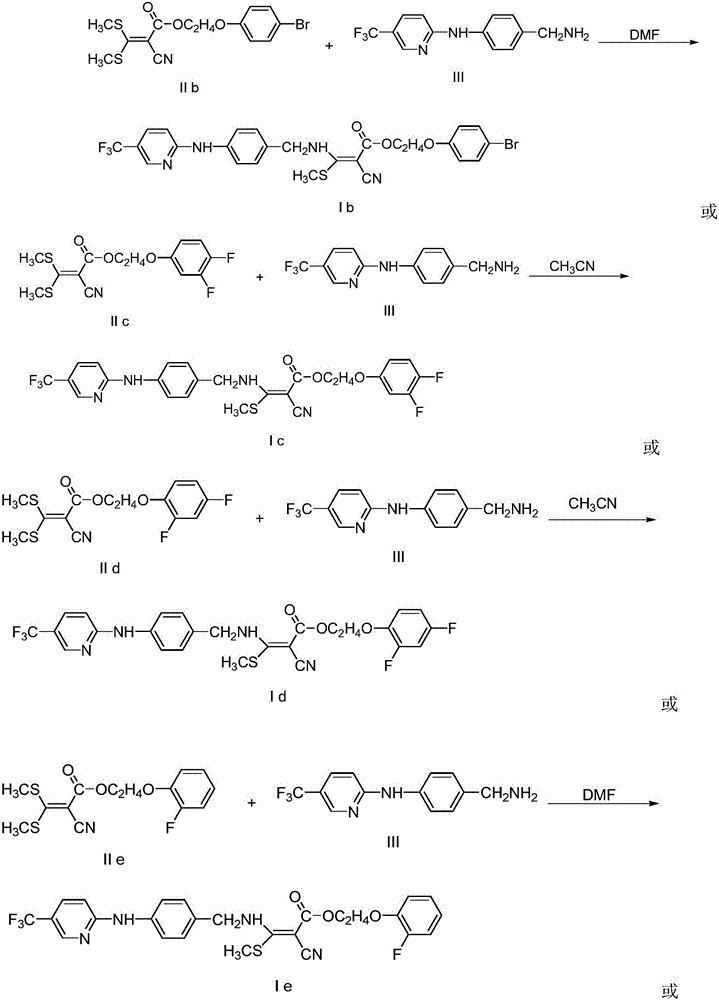
Embodiment 2
[0029]
[0030] In a 50mL round bottom flask, add 4mmolIIb, 4.4mmolIII and 20mL DMF, stir at room temperature for 6 hours, pour the reaction solution into 20mL ice water, extract with 30mL ethyl acetate, combine the organic layers, wash with water, dry over anhydrous magnesium sulfate, pump Filtration, evaporation of the solvent under reduced pressure, separation and purification by column chromatography gave the target compound Ib with a yield of 58%; 1 HNMR (400MHz, d 6 -DMSO): δ10.19(s,1H,NH),9.67(s,1H,NH),8.48(s,1H,Py-H),7.86(d,J=8.0Hz,1H,Py-H) ,7.70(d,J=8.0Hz,2H,Ar-H),7.44(d,J=8.0Hz,2H,Ar-H),7.27(d,J=12.0Hz,2H,Ar-H),6.93 -6.96(m,3H,Ar-HandPy-H),4.75(s,2H,CH 2 ),4.42(t,J=4.0Hz,2H,CH 2 ), 4.21(t, J=4.0Hz, 2H, CH 2 ),2.62(s,3H,SCH 3 ).
Embodiment 3
[0032]
[0033] In a 50mL round bottom flask, add 5mmolIIc, 6mmolIII and 25mL acetonitrile, heat to reflux for 6 hours, evaporate the solvent to dryness, add 20mL ice water to the reaction residue, extract with 30mL ethyl acetate, combine the organic layers, wash with water, Dry over magnesium sulfate, filter with suction, evaporate the solvent under reduced pressure, separate and purify by column chromatography to obtain the target compound Ic with a yield of 55%; 1 HNMR (400MHz, d 6 -DMSO): δ10.18(s,1H,NH),9.66(s,1H,NH),8.48(s,1H,Py-H),7.86(d,J=8.0Hz,1H,Py-H) ,7.70(d,J=8.0Hz,2H,Ar-H),7.30-7.37(m,1H,Ar-H),7.26(d,J=8.0Hz,2H,Ar-H),6.81-7.14( m,3H,Ar-HandPy-H),4.75(d,J=4.0Hz,2H,CH 2 ),4.42(t,J=4.0Hz,2H,CH 2 ), 4.22(t, J=4.0Hz, 2H, CH 2 ),2.62(s,3H,SCH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com