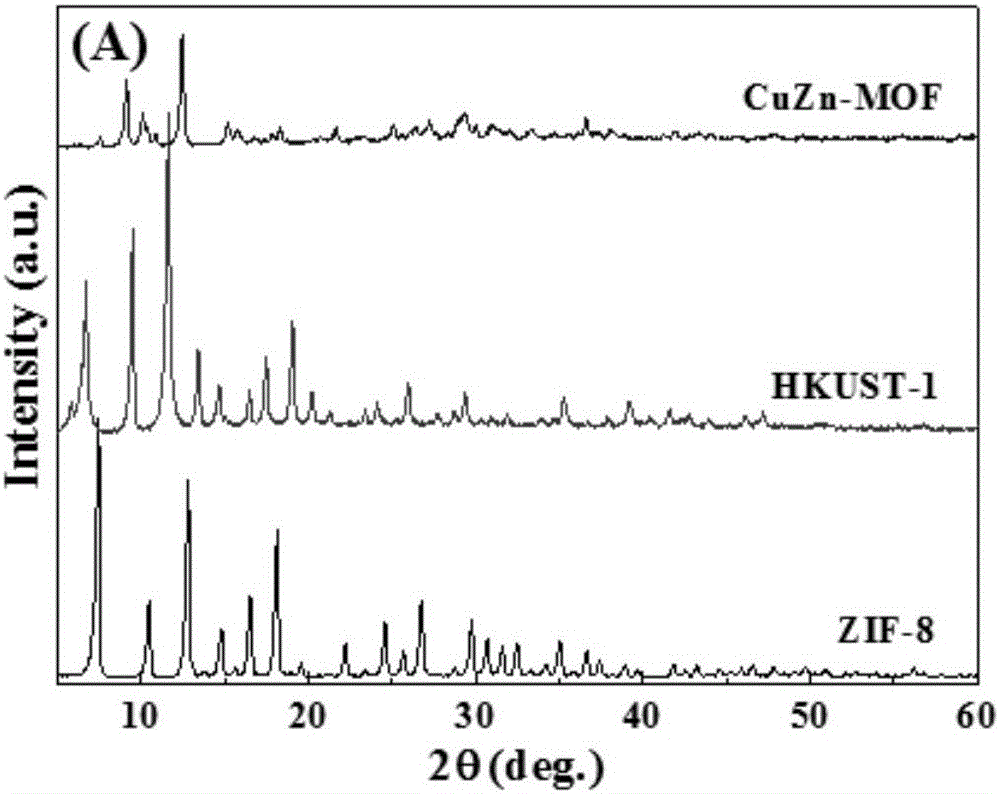
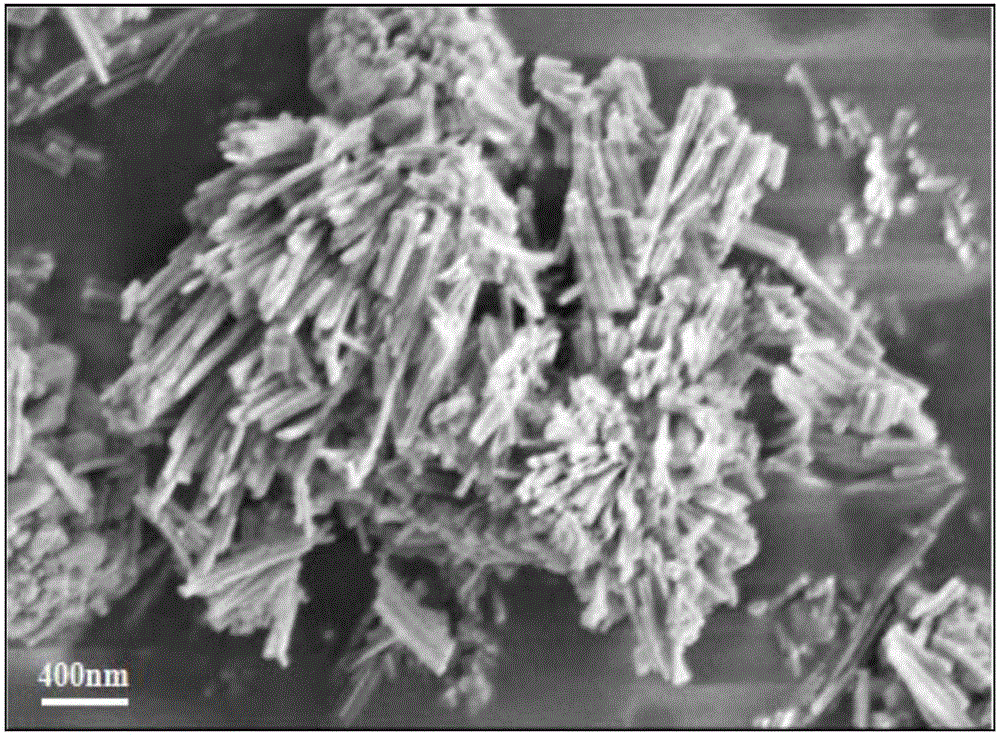
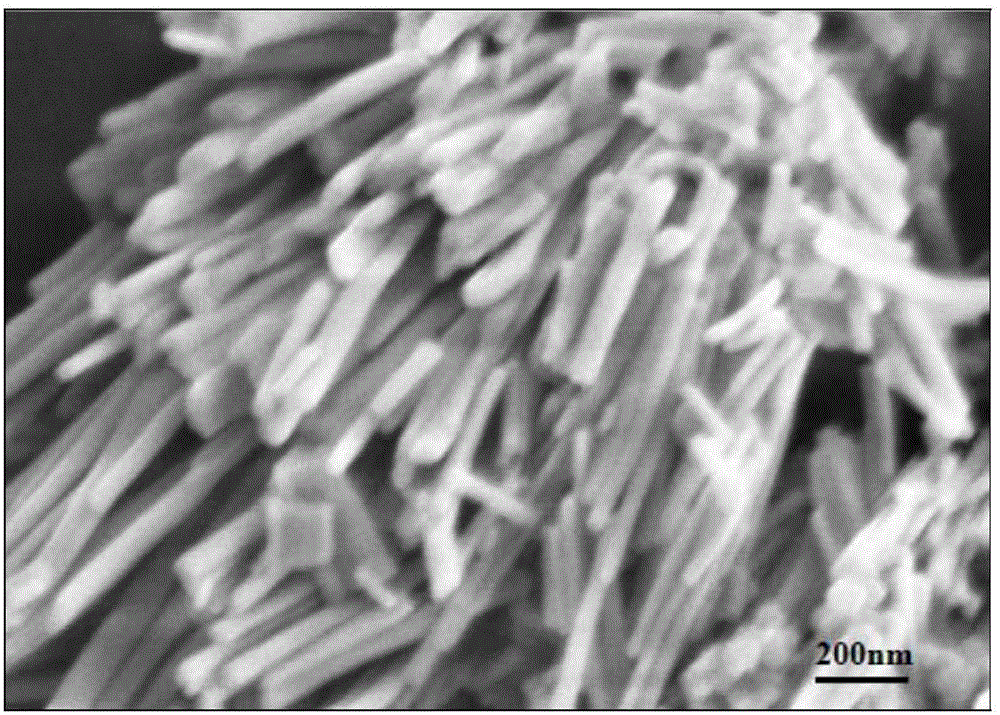
CuZn bi-metal organic framework material and preparing method thereof
An organic framework and bimetallic technology, applied in the field of organic-inorganic hybrid materials, can solve the problems of long preparation time and low replacement rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 2.97g zinc nitrate hexahydrate was dissolved in 15mL deionized water to form solution A, 1.64g dimethylimidazole was dissolved in 18.8g ammonia water (NH 3 The mass concentration is 28%) to form solution B, solution B is added dropwise in solution A, in the mixed solution Zn:Hmim:NH 3 :H 2 O molar ratio 1:2:32:157. After stirring at room temperature for 10 min, the resulting solid was centrifuged, washed with deionized water until the supernatant pH ≈ 7, then centrifuged and dried at 60°C for 12 h to obtain a Zn-containing metal-organic framework material ZIF-8. The ZIF-8 prepared by weighing 0.5g and 0.532g copper nitrate trihydrate were dissolved in deionized water (H 2 O): ethanol: N, N-dimethylformyl (DMF) = 1:1:1 (volume ratio) mixed solution (45mL), stirred until a uniform suspension was formed, then added 0.256g1,3,5 -Benzenetricarboxylic acid, stirred for 15min, transferred the solution to a 100mL stainless steel reaction kettle with a polytetrafluoroethylene...
Embodiment 2
[0048] 2.97g zinc nitrate hexahydrate was dissolved in 15mL deionized water to form solution A, 1.64g dimethylimidazole was dissolved in 18.8g ammonia water (NH 3 The mass concentration is 28%) to form solution B, solution B is added dropwise in solution A, in the mixed solution Zn:Hmim:NH 3 :H 2 O molar ratio 1:2:32:157. After stirring at room temperature for 10 min, the resulting solid was centrifuged, washed with deionized water until the supernatant pH ≈ 7, then centrifuged and dried at 60°C for 12 h to obtain a Zn-containing metal-organic framework material ZIF-8. The ZIF-8 prepared by weighing 0.5g and 1.596g copper nitrate trihydrate were dissolved in deionized water (H 2 O): ethanol: N, N-dimethylformyl (DMF) = 1:0.5:0.2 (volume ratio) mixed solution (51mL), stirred until a uniform suspension was formed, then added 0.512g1,3,5 -Benzenetricarboxylic acid, stirred for 15min, transferred the solution to a 100mL stainless steel reactor equipped with a polytetrafluoroeth...
Embodiment 3
[0050] 2.97g zinc nitrate hexahydrate was dissolved in 15mL deionized water to form solution A, 1.64g dimethylimidazole was dissolved in 18.8g ammonia water (NH 3 The mass concentration is 28%) to form solution B, solution B is added dropwise in solution A, in the mixed solution Zn:Hmim:NH 3 :H 2 O molar ratio 1:2:32:157. After stirring at room temperature for 10 min, the resulting solid was centrifuged, washed with deionized water until the supernatant pH ≈ 7, then centrifuged and dried at 60°C for 12 h to obtain a Zn-containing metal-organic framework material ZIF-8. The ZIF-8 prepared by weighing 0.5g and 1.064g copper nitrate trihydrate were dissolved in deionized water (H 2 O): Ethanol: N, N-dimethylformyl (DMF) = 1:0.5:2 (volume ratio) mixed solution (87.5mL), stirred until a uniform suspension was formed, then added 0.384g1,3, 5-Benzenetricarboxylic acid, stirred for 15min, transferred the solution to a 100mL stainless steel reactor equipped with polytetrafluoroethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com