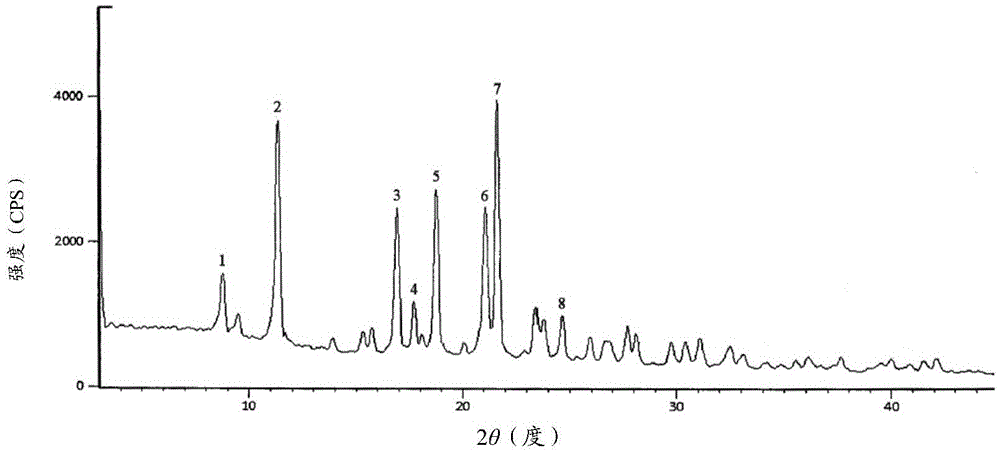
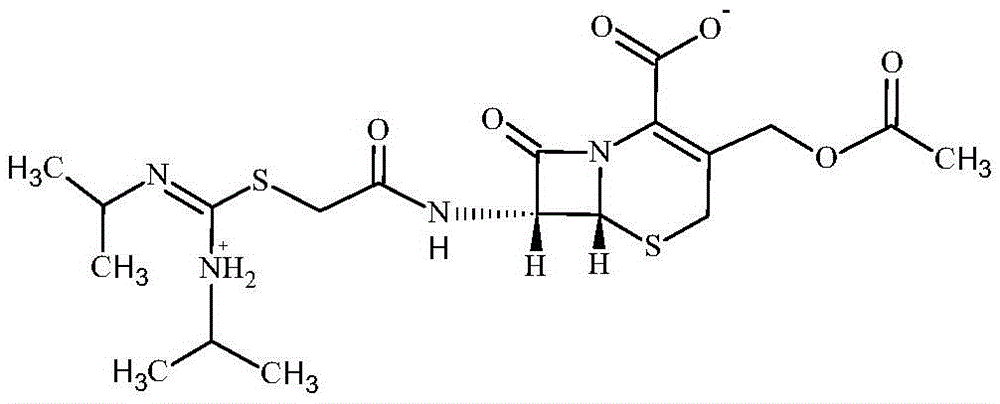
Novel-crystal-form cefathiamidine compound adopting crystal product molecular assembly and form optimization technology in particle process and preparation
A technology of cefathiamidine and compounds, applied in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., can solve the problem of poor stability of conventional crystal forms of cefathiamidine, poor selection of solvent systems, and poor color grades And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: Preparation of Cefathiamidine New Crystal Form Compound
[0024] (1) Add 5.0 g of N,N'-diisopropylsulfur into 120 mL of dichloromethane, stir to dissolve, add 10.1 g of bromoacetamidocephalosporanic acid and 3 g of triethylamine, and keep the reaction at 45°C for 1.5 h. The reaction temperature was lowered to 5° C. and crystallization was carried out for 1 h to form a crystallization solution. Suction filtration, washing with acetone twice, 20ml each time, and vacuum drying at 40°C gave 9.4g of crude cefathiamidine.
[0025] (2) Add 50ml of water for injection and 5.0g of cefathiamidine into the reactor, stir to dissolve, add 0.1g of medicinal charcoal, stir and decolorize for 30min, filter the decolorization solution with a sterilizing filter, and adjust the pH value of the filtrate with hydrochloric acid 4.1. Add 200ml of isopropanol to the filtrate, cool to 0-5°C, stir for 1 hour, crystallize out, wash with acetone twice, 50ml each time, use ethanol as...
Embodiment 2
[0032] Example 2: Preparation of Cefathiamidine New Crystal Form Compound
[0033] (1) Add 10.2g of N,N'-diisopropylsulfur into 120mL of dichloromethane, stir to dissolve, add 10.1g of bromoacetamidocephalosporanic acid and 3.1g of triethylamine, and keep the reaction at 50°C for 1.5h , the reaction temperature was lowered to 0° C. for 1 h, and the crystallization liquid was formed. Suction filtration, washing with acetone twice, 20ml each time, and vacuum drying at 40°C to obtain 10.5g of crude cefathiamidine.
[0034] (2) Add 50ml of water for injection and 5.5g of cefathiamidine into the reactor, stir to dissolve, add 0.1g of medicinal charcoal, stir and decolorize for 30min, filter the decolorizing solution with a sterilizing filter, and adjust the pH value of the filtrate with hydrochloric acid 3.5. Add 200ml of isopropanol to the filtrate, lower the temperature to 0-5°C, stir for 1 hour, precipitate crystals, wash with acetone twice, 50ml each time, use ethanol as solve...
Embodiment 3
[0036] Example 3: Preparation of Cefathiamidine New Crystal Form Compound
[0037] (1) Add 5.4g of N,N'-diisopropylsulfur into 120mL of dichloromethane, stir to dissolve, add 15.3g of bromoacetamidocephalosporanic acid and 4.0g of triethylamine, and keep it at 50°C for 1.5h , the reaction temperature was lowered to 0° C. for 1 h, and the crystallization liquid was formed. Suction filtration, washing with acetone twice, 20ml each time, and vacuum drying at 40°C gave 5.4g of crude cefathiamidine.
[0038] (2) Add 50ml of water for injection and 5.1g of cefathiamidine into the reactor, stir and dissolve, add 0.1g of medicinal charcoal, stir and decolorize for 30min, filter the decolorizing solution with a sterilizing filter, and adjust the pH value of the filtrate with hydrochloric acid 5.5. Add 200ml of isopropanol to the filtrate, cool down to 0-5°C, stir for 1 hour, crystallize out, wash with acetone twice, 50ml each time, use ethanol as solvent, and recrystallize to obtain 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com