Heterocyclic imide derivative containing bisamide structure and preparation method and application thereof
A technology for heterocyclic imines and derivatives, which is applied in the field of heterocyclic imine derivatives and their preparation, can solve the problems of serious cross-resistance, limited application space of chemical pesticides, proliferation of pest resistance problems, etc., and achieves easy availability of raw materials. , the preparation process is simple and easy, and the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
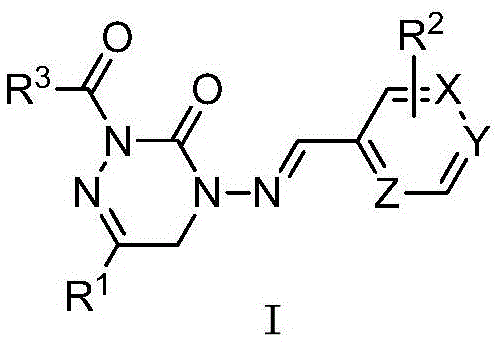
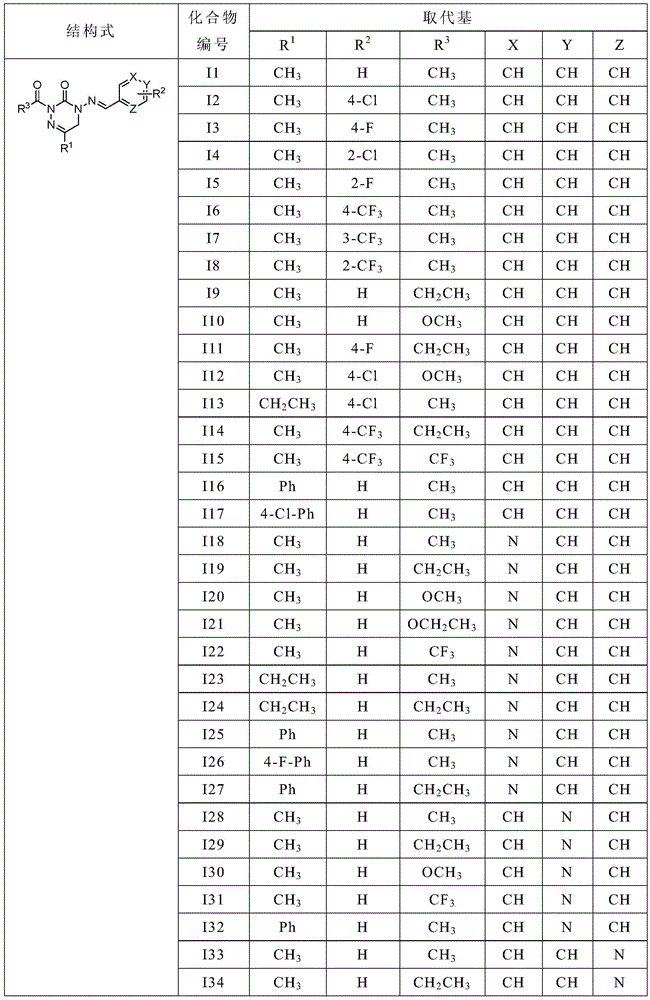
[0031] The structural formula of part of the heterocyclic imine derivatives containing bisamide structure of the present invention is one of the specific compounds listed in Table 1:
[0032] Table 1: Representative compounds shown in structural formula I
[0033]
[0034]
Embodiment 2
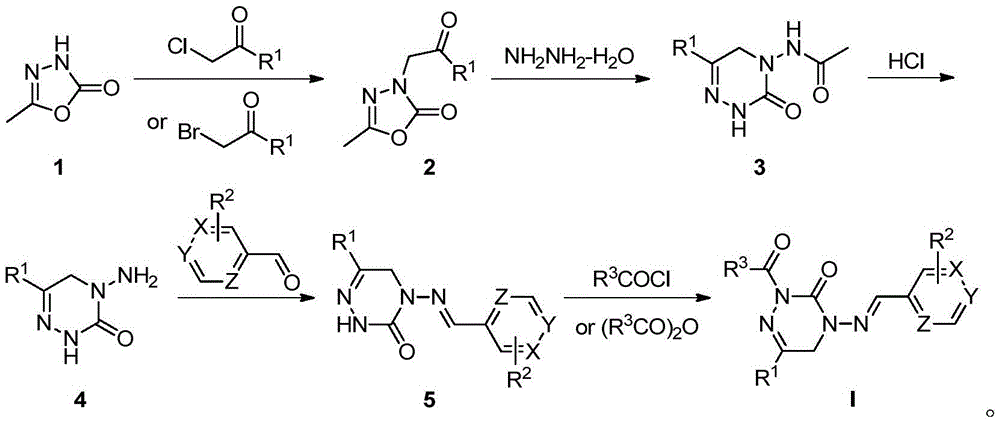
[0036] Numbering is the preparation of the compound of I1 among the embodiment 1, and its reaction formula is:
[0037]
[0038] Its preparation method comprises the following steps:
[0039] 1) Using 5-methyl-1,3,4-oxadiazol-2(3H)-one (compound 1) and chloroacetone as starting materials to react under the condition of sodium hydroxide as base to obtain alkylation Intermediate 2a;
[0040] 2) Dissolve 10 mmol of the alkylation intermediate 2a in ethanol, add 12 mmol of hydrazine hydrate dropwise under stirring, slowly heat up to reflux after addition, continue the reaction, follow TLC until the reaction is complete, and obtain the heterocyclic intermediate after conventional post-treatment 3a;
[0041] 3) The heterocyclic intermediate 3a is deacetylated under acidic conditions to obtain the amino-substituted triazone heterocyclic intermediate 4a;
[0042] 4) Dissolve 10mmol of amino-substituted triazone heterocyclic intermediate 4a in 15mL of ethanol, add 10mmol of benza...
Embodiment 3
[0046] Numbering is the preparation of the compound of I10 among the embodiment 1, and its reaction formula is:
[0047]
[0048] Its preparation method comprises the following steps:
[0049] Steps 1) to 4) refer to Example 2 to obtain the imine intermediate 5aa;
[0050] 5) Weigh 2 mmol of imine compound 5aa, suspend it in 20 mL of anhydrous THF, then add 2.2 mmol of potassium tert-butoxide in batches, stir at room temperature for about 30 min, and then dropwise add 2.2 mmol of methyl chloroformate in THF Solution, dropwise, continue to stir the reaction, TLC traces the reaction is complete, add an appropriate amount of water to quench the reaction, use dichloromethane to extract three times, the organic phase is washed with brine and water, dried and concentrated to obtain the crude product, and then separated and purified by column chromatography to obtain the target product I10.
[0051] The physical and chemical properties of the target compound I10: 1 HNMR (CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com