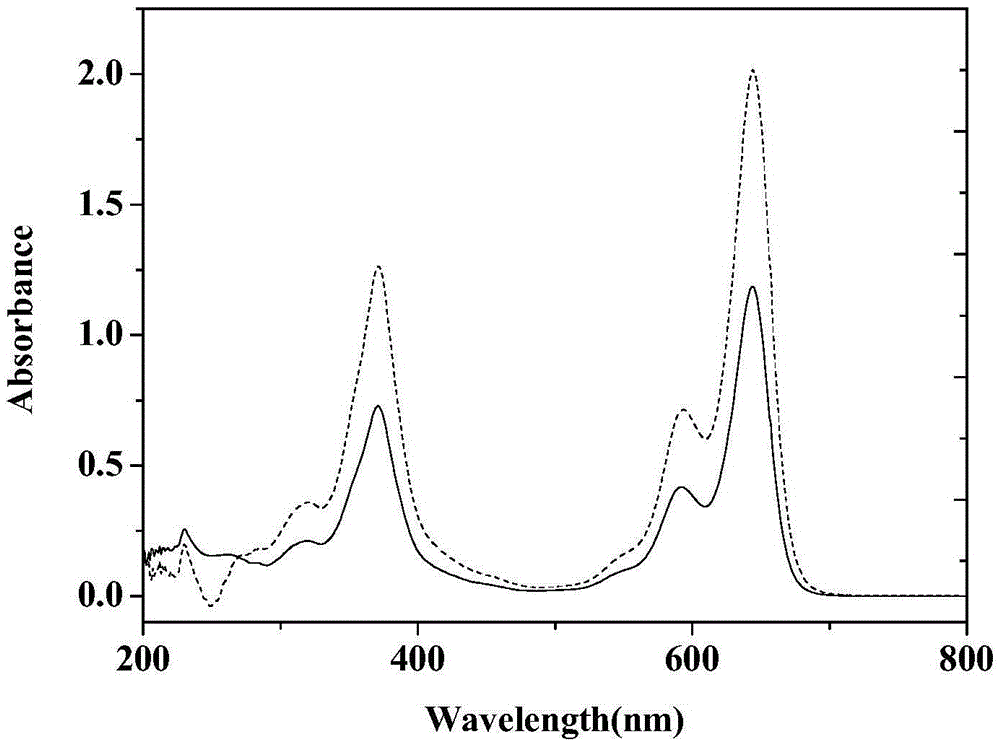
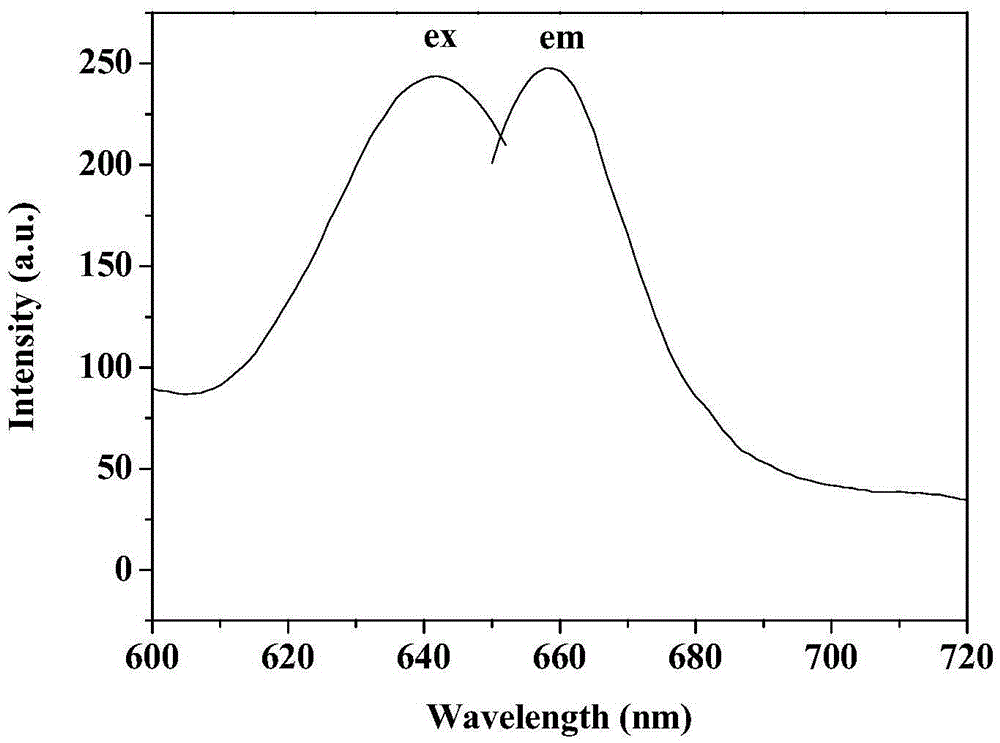
Near-infrared dye and preparation method thereof
A technology of red light and dyes, which is applied in the field of near-red dyes and their preparation, and can solve the problems of limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0019] Embodiment 1.1 (preferred embodiment)
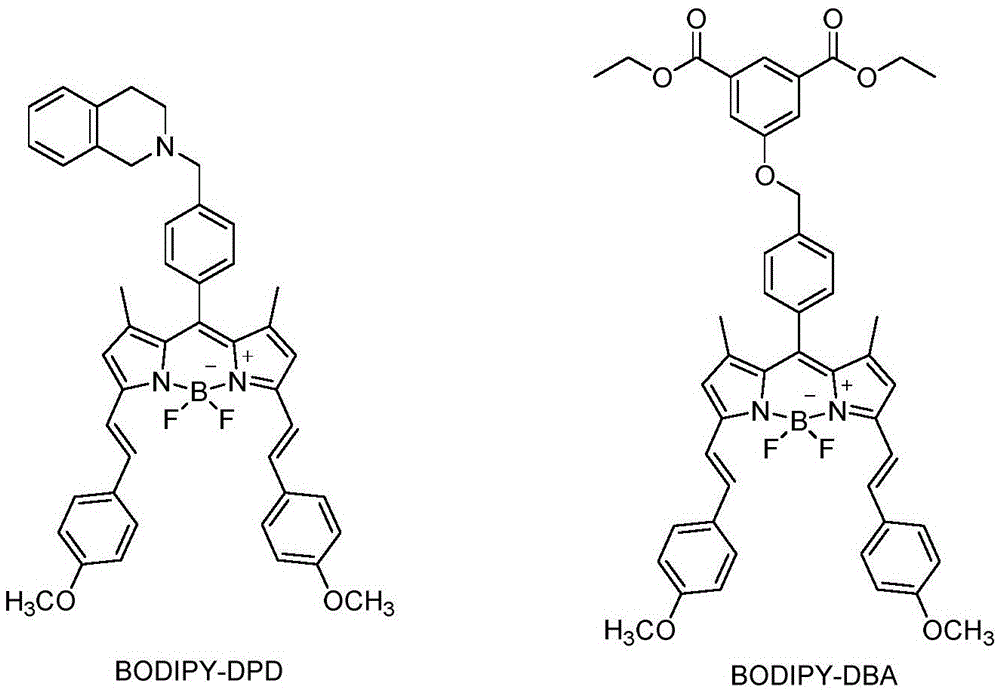
[0020] Compound 1,7-Dimethyl-3,5-(bis(4-methoxyphenyl-2-enyl))-8-(2-(1,2,3-trihydroisoquinoline)) -4-boron-3a,4a-dipyrrole (abbreviated as BODIPY-DPD)
[0021] BODIPY-C (1.03mmol) and 1,2,3,4-tetrahydroisoquinoline (1.545mmol) were mixed at a molar ratio of 1:1.5, then 25mL of tetrahydrofuran was added, followed by K 2 CO 3 (1.236mmol), KI (34.2mg, 0.206mmol) and hexadecylcrown ether (0.1mg); they were mixed and heated to 65°C in a water bath for 6 hours, the reaction solution was concentrated and dissolved in 10mL of dichloromethane and filtered, and the solution was set up The water trap was refluxed, followed by adding 4-methoxybenzaldehyde (2.575 mmol), adding 20 mL of toluene, followed by adding 4-methylbenzenesulfonic acid and piperidine, 4-methylbenzenesulfonic acid (0.206 mmol) and piperidine The ratio of (0.515mmol) to BODIPY-C is 0.2:1 and 0.5:1 respectively. After mixing, heat to 120°C and react for 8 hours. Saturat...
Embodiment 12
[0022] Embodiment 1.2 (preferred embodiment)
[0023] BODIPY-C (1.03mmol) and ethyl 5-hydroxyisophthalate (1.545mmol) were mixed at a molar ratio of 1:1.5, then 25mL of tetrahydrofuran was added, followed by K 2 CO 3 (1.236mmol) KI (0.206mmol) hexadecyl crown ether (0.1mg); where mixed and heated to 65°C in a water bath for 6 hours. Concentrate the reaction solution and dissolve it with 10ml of dichloromethane, then filter the solution to set up a water trap to reflux, then add 4-methoxybenzaldehyde (351.2mg, 2.575mmol), add 20mL of toluene, then add 4-methylbenzenesulfonic acid and piperazine The ratios of pyridine, 4-methylbenzenesulfonic acid (0.206mmol) and piperidine (0.515mmol) to BODIPY-C are 0.2:1 and 0.5:1 respectively. After mixing, heat to 140°C and react for 8 hours. After the reaction , concentrated the reaction liquid, added 20mL of dichloromethane, extracted and washed with 3×30mL saturated brine, extracted the aqueous phase with 3×20mL of dichloromethane, com...
Embodiment 21
[0026] BODIPY-C (1.03mmol) and 1,2,3,4-tetrahydroisoquinoline (0.515mmol) were mixed at a molar ratio of 2:1, then 25mL of tetrahydrofuran was added, followed by K 2 CO 3 (1.236mmol) KI (34.2mg, 0.206mmol) hexadecylcrown ether (0.1mg); the mixture was heated to 65°C in a water bath for 6 hours, the reaction solution was concentrated and dissolved in 10mL of dichloromethane and then filtered, and the solution was set up to divide the water Then add 4-methoxybenzaldehyde (2.575mmol), add 20mL toluene, then add 4-methylbenzenesulfonic acid and piperidine, 4-methylbenzenesulfonic acid (0.206mmol) and piperidine (0.515 The ratio of mmol) to BODIPY-C is 0.2:1 and 0.5:1 respectively. After mixing, heat to 120°C and react for 8 hours. After the reaction, concentrate the reaction solution, add 20mL of dichloromethane, and use 3×30mL saturated brine After extraction and washing, the aqueous phase was extracted with 3×20 mL of dichloromethane, the organic phases were combined, dried ove...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com