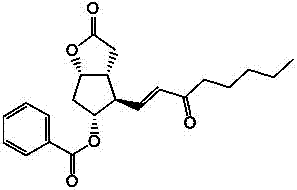
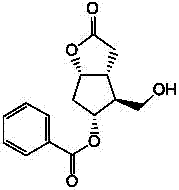
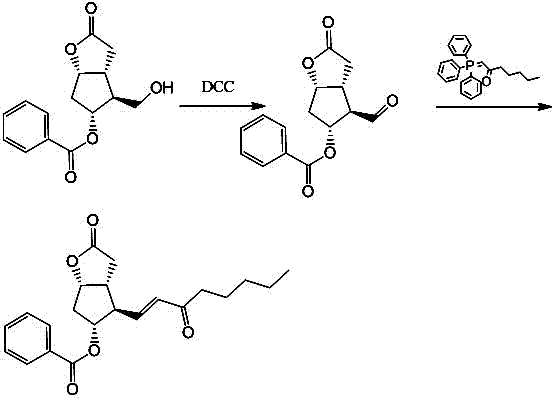
Preparation method of 15-ketone
An organic solvent and benzoyl technology, applied in the field of chemical pharmaceuticals, can solve the problems that the reaction temperature is difficult to control at -78°C, it is not suitable for industrial production, and the post-processing is cumbersome, so as to facilitate product precipitation, simplify operation, and shorten reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 40g of (-)-benzoyl corylide, 240ml of dimethyl sulfoxide, and 480ml of dichloromethane into the reaction flask, add 100g of dicyclohexylcarbodiimide (DCC), stir, cool down to 15°C, add 12ml of pyridine, 6ml of trifluoroacetic acid, then stirred at 25°C for 4h; added 200ml of dichloromethane, cooled to 15°C to obtain reaction solution A, and added dropwise dilute hydrochloric acid prepared from 15ml of concentrated hydrochloric acid and 1000ml of water to reaction solution A , stirred for 20 minutes, filtered, washed the filter cake with 100ml of dichloromethane, separated into layers, extracted the water layer with 400ml×2 dichloromethane, combined the organic layer, washed with 500ml×2 water, added 100g of anhydrous sodium sulfate to dry and dehydrate, filtered , to obtain (-)-benzoyl corylide aldehyde solution, add 100g 1-triphenylphosphine-2-heptanone (Wittig reagent) to the solution, stir at 20-30°C for 8h to carry out Wittig reaction , to obtain the reaction solu...
Embodiment 2
[0023] Add 40g of (-)-benzoyl corylide, 240ml of dimethyl sulfoxide, and 510ml of chloroform into the reaction flask, add 100g of dicyclohexylcarbodiimide (DCC), stir, cool down to 10°C, add 12ml of pyridine, 6ml of trifluoroacetic acid, then stirred at 25°C for 6h; added 180ml of chloroform, cooled to 10°C to obtain reaction solution A, and added dropwise dilute hydrochloric acid prepared from 15ml of concentrated hydrochloric acid and 1000ml of water to reaction solution A , stirred for 20min, filtered, washed the filter cake with 100ml of chloroform, separated into layers, extracted the water layer with 400ml×2 chloroform, combined the organic layer, washed with 500ml×2 water, added 100g of anhydrous magnesium sulfate to dry and dehydrate, filtered , to obtain (-)-benzoyl corylide aldehyde solution, add 100g 1-triphenylphosphine-2-heptanone (Wittig reagent) to the solution, stir at 20-30°C for 6h to carry out Wittig reaction , to obtain the reaction solution B, evaporate th...
Embodiment 3
[0025] Add 40g of (-)-benzoyl corylide, 240ml of dimethyl sulfoxide, and 550ml of dichloromethane into the reaction flask, add 100g of dicyclohexylcarbodiimide (DCC), stir, cool down to 15°C, add 10ml of pyridine, 8ml of trifluoroacetic acid, then stirred at 20°C for 4h; add 100ml of dichloromethane and cool down to 15°C to obtain reaction solution A, add dropwise dilute hydrochloric acid prepared by 15ml of concentrated hydrochloric acid and 1000ml of water into reaction solution A , stirred for 20min, filtered, washed the filter cake with 100ml of dichloromethane, layered, extracted the water layer with 400ml×2 dichloromethane, combined the organic layer, washed with 500ml×2 water, added 100g of anhydrous potassium carbonate to dry and dehydrate, filtered , to obtain (-)-benzoyl corylide aldehyde solution, add 120g 1-triphenylphosphine-2-heptanone (Wittig reagent) to the solution, stir at 20-30°C for 12h to carry out Wittig reaction , to obtain the reaction solution B, evapo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com