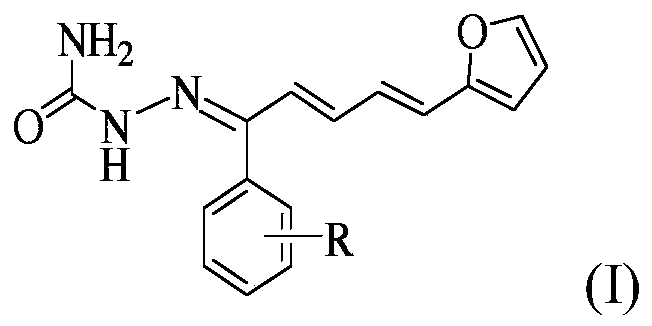
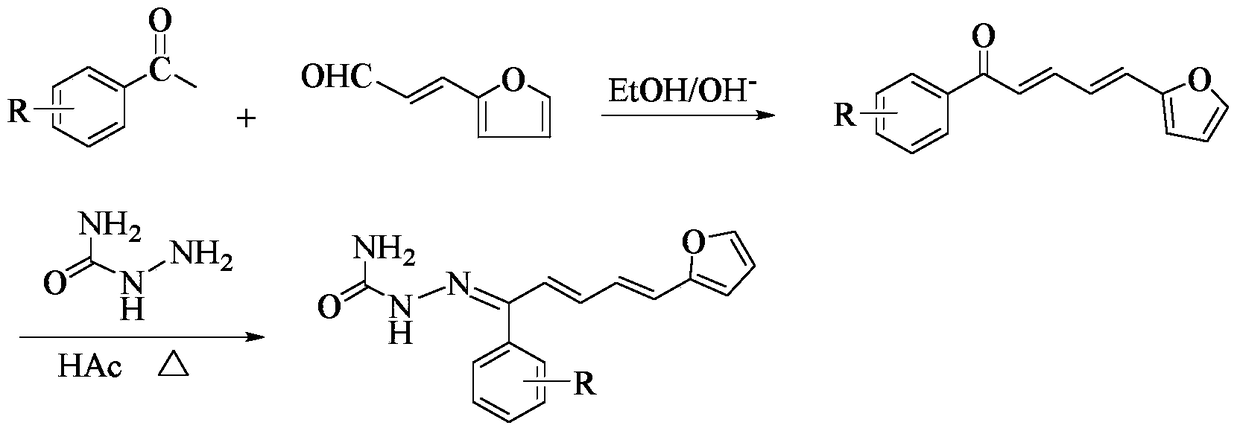
1-(2-furyl)-1,3-pentadiene compound and its preparation method and application
The technology of a compound, pentadiene, is applied in the field of pesticides to achieve good control effect, simple structure and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] compound (C 16 h 14 o 2 N 3 Br) preparation
[0033] (1) Synthesis of intermediate 1-(4-bromophenyl)-5-(2-furyl)-2,4-pentadien-1-one
[0034] Dissolve 0.02 mol of 4-bromoacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. While stirring in an ice bath, slowly drop the mixture of 0.02mol 2-furan acrolein and 20mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-bromophenyl)-5-(2-furyl)-2,4-pentadien-1-one.
[0035] (2) Synthesis of the target compound
[0036] 0.015 mol of semicarbazide was dissolved in 20 mL of 90% ethanol, and 1.5 ...
Embodiment 2
[0039] compound (C 22 h 19 o 2 N 3 ) preparation
[0040] (1) Synthesis of intermediate 1-(4-biphenyl)-5-(2-furyl)-2,4-pentadien-1-one
[0041] Dissolve 0.02 mol of biphenyl monoethyl ketone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. While stirring in an ice bath, slowly drop the mixture of 0.02mol 2-furan acrolein and 20mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-biphenyl)-5-(2-furyl)-2,4-pentadien-1-one.
[0042] (2) Synthesis of the target compound
[0043] 0.015 mol of semicarbazide was dissolved in 20 mL of 90% ethanol, and 1.5 mL...
Embodiment 3
[0046] compound (C 16 h 14 o 2 N 3 Br) preparation
[0047] (1) Synthesis of intermediate 1-(3-bromophenyl)-5-(2-furyl)-2,4-pentadien-1-one
[0048] Dissolve 0.02 mol of 3-bromoacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. While stirring in an ice bath, slowly drop the mixture of 0.02mol 2-furan acrolein and 20mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(3-bromophenyl)-5-(2-furyl)-2,4-pentadien-1-one.
[0049] (2) Synthesis of the target compound
[0050] 0.015 mol of semicarbazide was dissolved in 20 mL of 90% ethanol, and 1.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com