Synthesis method for 2,2-dimethyl-5-(4-bromophenyl)-6-amino-3-hexanone
A synthesis method, technology of bromophenyl, applied in the field of synthesis of 2,2-dimethyl-5--6-amino-3-hexanone, can solve backward technology, difficult to manage, and no ideal industrial production method And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
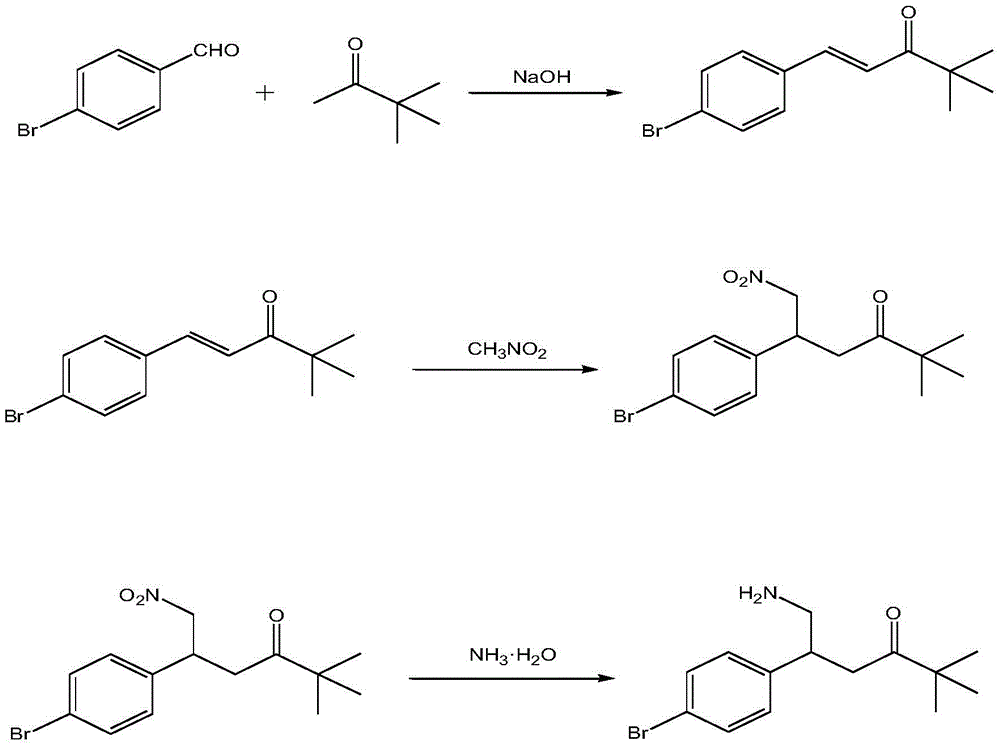
[0015] Measure 0.2mol pinacolone, 3g sodium hydroxide, pour it into 500mL ethanol solution with a mass concentration of 80%, then transfer it into a water bath, heat it to 50°C, place it on a magnetic stirrer, and stir at a speed of 200r / min 20min; Pour 0.2mol of p-bromobenzaldehyde into a 100mL beaker, add 50mL of ethanol solution with a mass concentration of 80%, stir evenly with a glass rod, add it dropwise to the above mixture, control the drop rate, Make it dropwise within 30min, and continue to stir for 40min; put the above mixture into a rotary evaporator, remove the ethanol by rotary evaporation, then place it in an ice-water bath, and let the ice bath stand for 1h until the precipitate no longer separates out. The material was dried and used for later use; 15mmol of the obtained precipitate was mixed with 50mL of absolute ethanol and placed in a 100mL beaker, stirred and dissolved with a glass rod for 10min, then 70mmol of nitromethane and 1mmol of potassium carbonate ...
example 2
[0017] Measure 0.2mol of pinacolone and 4g of sodium hydroxide, pour it into 500mL of ethanol solution with a mass concentration of 80%, then transfer it into a water bath, heat it to 60°C, place it on a magnetic stirrer, and stir at a speed of 250r / min 25min; Pour 0.2mol p-bromobenzaldehyde into a 100mL beaker, add 60mL of ethanol solution with a mass concentration of 80%, stir evenly with a glass rod, add it dropwise to the above mixture, control the drop rate, Make it dropwise within 35min, and continue to stir for 50min; put the above mixture into a rotary evaporator, remove the ethanol by rotary evaporation, then place it in an ice-water bath, and let it stand in the ice bath for 1h until the precipitate no longer precipitates. 18mmol of the obtained precipitate was mixed with 55mL of absolute ethanol and put into a 100mL beaker, stirred and dissolved with a glass rod for 15min, then 75mmol of nitromethane and 2mmol of potassium carbonate were added, and the temperature wa...
example 3
[0019] Measure 0.3mol pinacolone, 5g sodium hydroxide, pour it into 500mL ethanol solution with a mass concentration of 80%, then transfer it into a water bath, heat it up to 70°C, place it on a magnetic stirrer, and stir at a speed of 300r / min 30min; Pour 0.3mol of p-bromobenzaldehyde into a 100mL beaker, add 70mL of ethanol solution with a mass concentration of 80%, stir evenly with a glass rod, add it dropwise to the above mixture, control the drop rate, Make it dropwise within 40min, and continue to stir for 60min; put the above mixture into a rotary evaporator, remove the ethanol by rotary evaporation, then place it in an ice-water bath, and let it stand for 2h in the ice bath until the precipitate no longer precipitates. 20mmol of the obtained precipitate was mixed with 60mL of absolute ethanol and put into a 100mL beaker, stirred and dissolved with a glass rod for 20min, then 80mmol of nitromethane and 3mmol of potassium carbonate were added, and the temperature was heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com