Preparing method for acanthopanax senticosus extract
A technology of Acanthopanax senticosus extract and Acanthopanax senticosus, applied in the field of preparation of Acanthopanax senticosus extract, which can solve the unfavorable industrial production of Acanthopanax senticosus preparations for medicinal safety, affecting the quality of Acanthopanax senticosus extract, and the extraction of active ingredients Low efficiency and other problems, to save the production cycle, shorten the extraction cycle, reduce the effect of loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
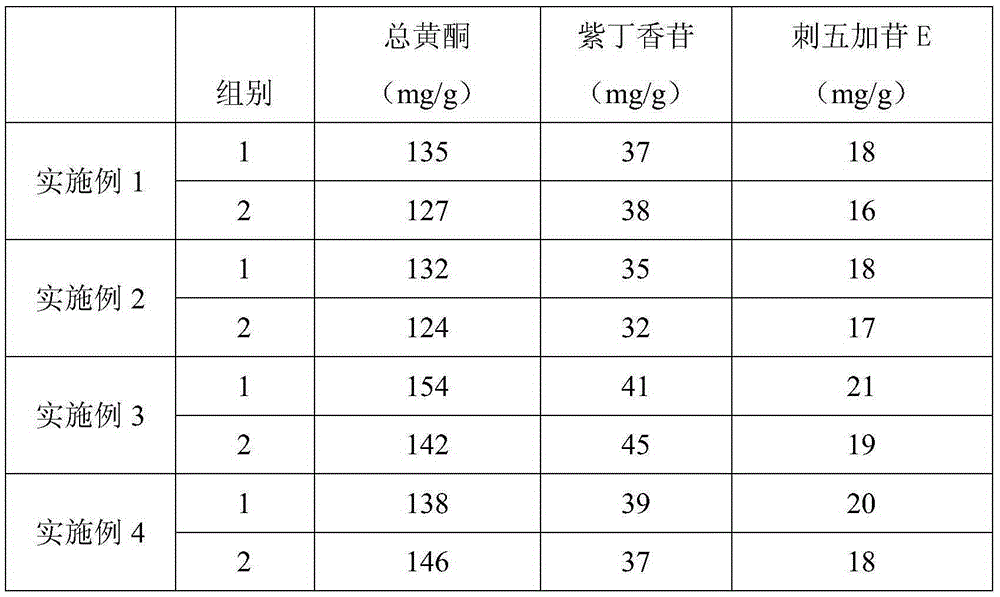
Embodiment 1
[0032] Embodiment 1: Preparation of Acanthopanax Acanthopanax Extract
[0033] 1) Extract 6kg of 40% ethanol with 3 volume times (18L) countercurrently to extract the medicinal materials of Acanthopanax senticosus. 1.20 (measured at 80°C);
[0034] 2) Dilute with 5 times the volume of purified water, stir evenly, filter, when the temperature of the filtrate is 30°C, adjust the pH value to 10.0 with 10% milk of lime, stir, then adjust the pH value to 5.0 with 10% sulfuric acid , continue to stir, stand still, filter the supernatant and recycle and concentrate to a relative density of 1.15 (measured at 80°C) to obtain a concentrated paste;
[0035] 3) Add ethanol with a concentration of 93% to make the ethanol concentration of the medicinal solution reach 75%, stir fully, let stand for 12 hours, filter the supernatant, recycle the ethanol from the filtrate until there is no alcohol smell, and concentrate to a relative density of 1.11 (measured at 80°C). );
[0036]4) Dilute w...
Embodiment 2
[0037] Embodiment 2: Preparation of Acanthopanax Acanthopanax Extract
[0038] 1) 8 times the volume (40L) of 70% ethanol countercurrently extracts 5kg of Acanthopanax senticosus medicinal materials, the countercurrent extraction flow rate is 35min / can, the extraction temperature is 80°C, and the extraction time is 0.5 hours, the combined extracts are concentrated to a relative density of 1.25 (measured at 80°C);
[0039] 2) Dilute with 10 times the volume of purified water, stir evenly, filter, and when the filtrate temperature is 50°C, adjust the pH value to 12.0 with 20% milk of lime, stir, and then adjust the pH value to 6.5 with 20% sulfuric acid , continue to stir, stand still, filter the supernatant and recycle and concentrate to a relative density of 1.25 (measured at 80°C) to obtain a concentrated paste;
[0040] 3) Add ethanol with a concentration of more than 98% to make the ethanol concentration of the medicinal solution reach 85%, fully stir, let stand for 24 hou...
Embodiment 3
[0042] Embodiment 3: Preparation of Acanthopanax Acanthopanax Extract
[0043] 1) 550% ethanol with 6 volume times (30L) countercurrently extracts 5kg of Acanthopanax acanthopanax medicinal material, the flow rate of countercurrent extraction is 30min / can, the extraction temperature is 50°C, and the extraction time is 1 hour, the extracts are combined and concentrated to a relative density of 1.25 (measured at 80°C);
[0044] 2) Dilute with 8 volume times of purified water, stir evenly, filter, and when the filtrate temperature is 40°C, adjust the pH value to 11 with 15% milk of lime, stir, and then adjust the pH value to 6 with 15% sulfuric acid , continue to stir, stand still, filter the supernatant and recycle and concentrate to a relative density of 1.25 (measured at 80°C) to obtain a concentrated paste;
[0045] 3) Add ethanol with a concentration of more than 95% to make the ethanol concentration of the medicinal solution reach 80%, fully stir, let stand for 16 hours, f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com