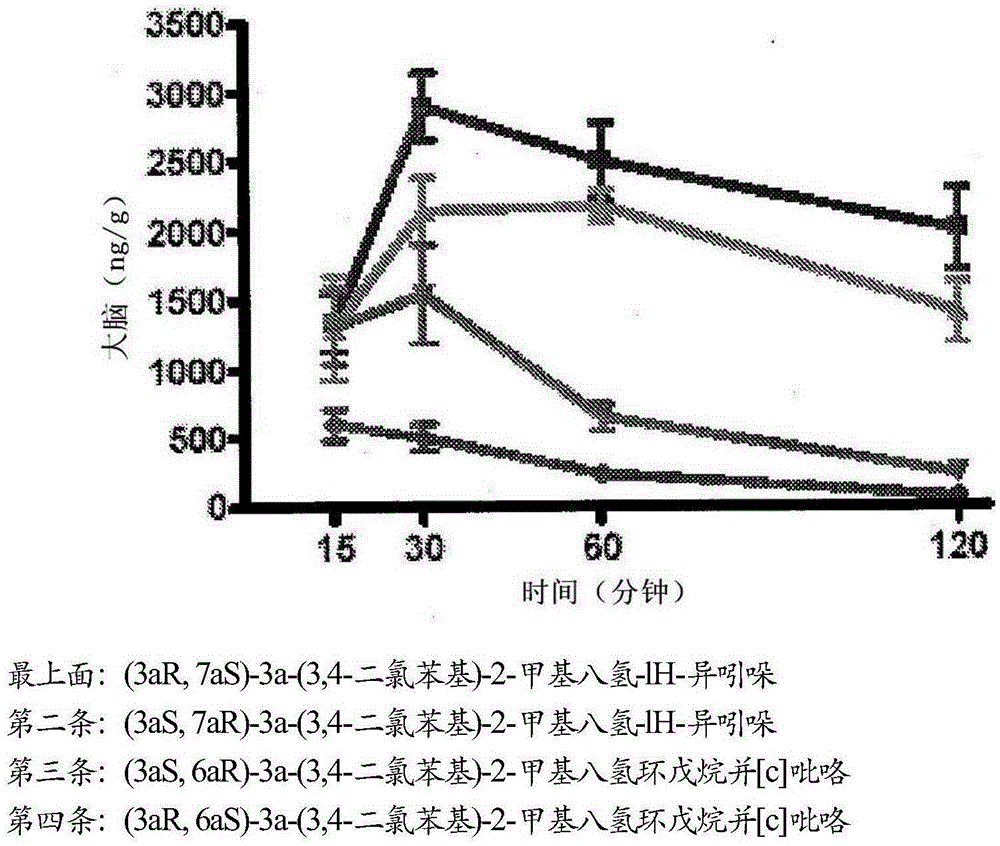
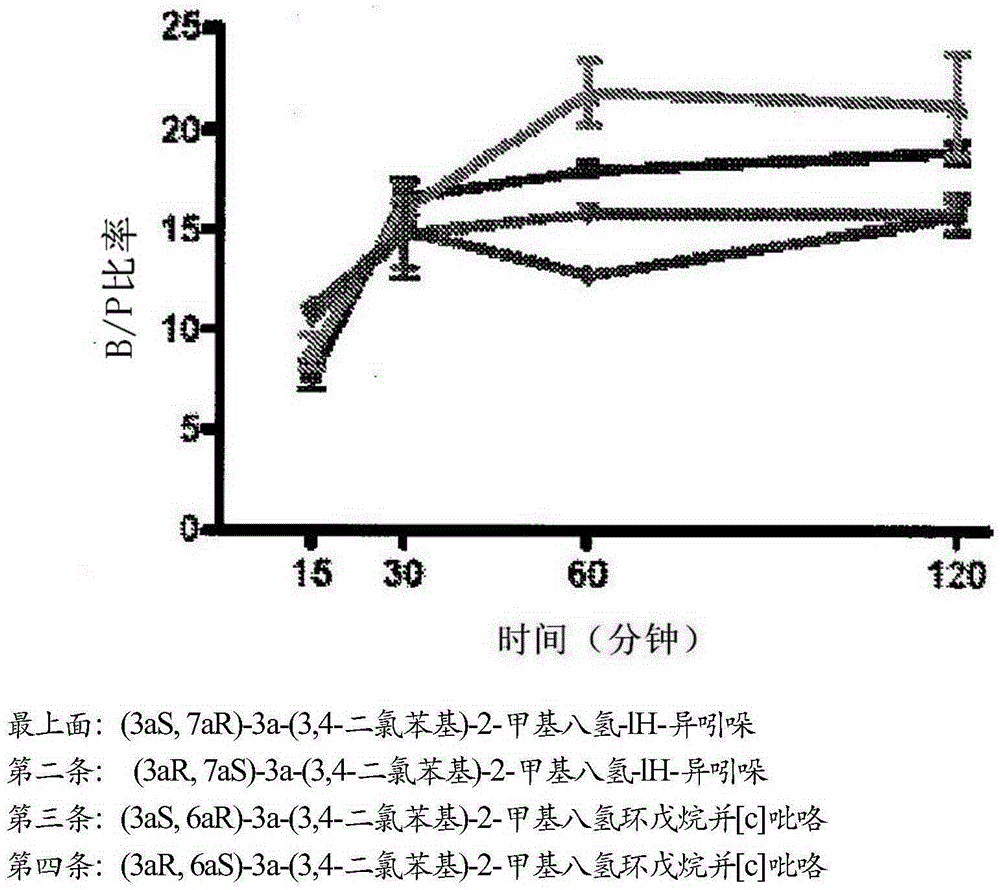
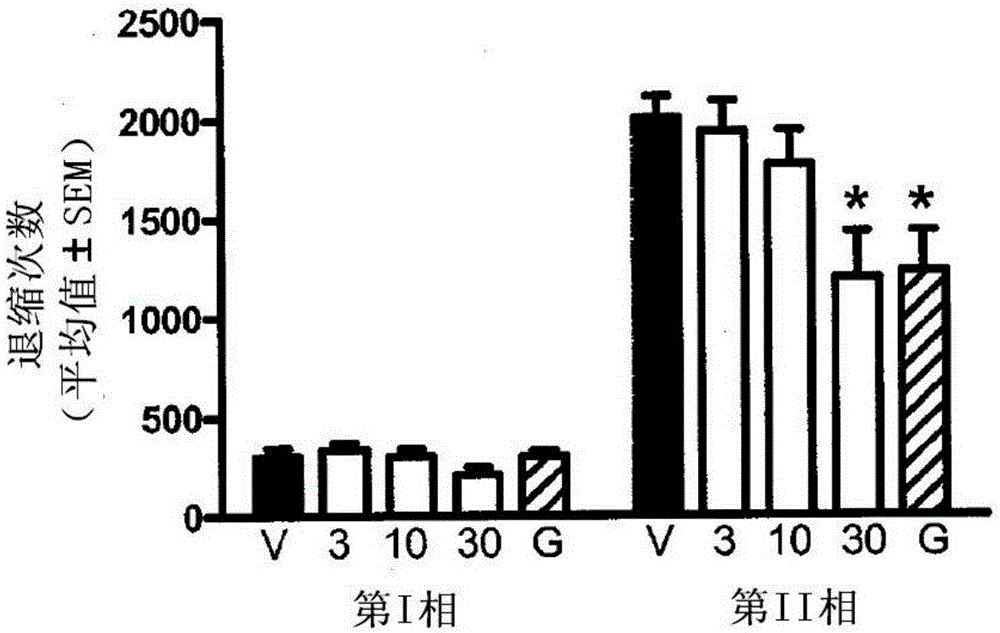
Triple reuptake inhibitors and methods of their use
A technology of solvates and compounds, applied in the field of compounds of triple reuptake inhibitors, can solve the problems of lag in therapeutic effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] 5.1 definition
[0020] Unless otherwise specified, the term "alkyl" used in the present invention refers to a linear or branched saturated monovalent hydrocarbon group, wherein the alkyl group may be optionally substituted by one or more substituents. The term "alkyl" also includes straight and branched chain alkyl groups unless otherwise specified. In certain embodiments, the alkyl group has 1 to 20(C 1-20 ), 1 to 15 (C 1-15 ), 1 to 12 (C 1-12 ), 1 to 10 (C 1-10 ), or 1 to 6 (C 1-6 ) straight-chain saturated monovalent hydrocarbon group of carbon atoms, or with 3 to 20 (C 3-20 ), 3 to 15 (C 3-15 ), 3 to 12 (C 3-12 ), 3 to 10 (C 3-10 ), or 3 to 6 (C 3-6 ) branched saturated monovalent hydrocarbon group of carbon atoms. The linear C used in the present invention 1-6 and branched C 3-6 Alkyl is also referred to as "lower alkyl". Embodiments of alkyl include, but are not limited to, methyl, ethyl, propyl (including all isomeric forms), n-propyl, isopropyl, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com