Preparation method of ester ether compound and catalyst applied to method
A technology of ester ethers and compounds, which is applied in the field of biofuels, can solve the problems of unfavorable industrial production, high production cost, and large reaction energy consumption, and achieve the effects of favorable industrial production, high selectivity, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
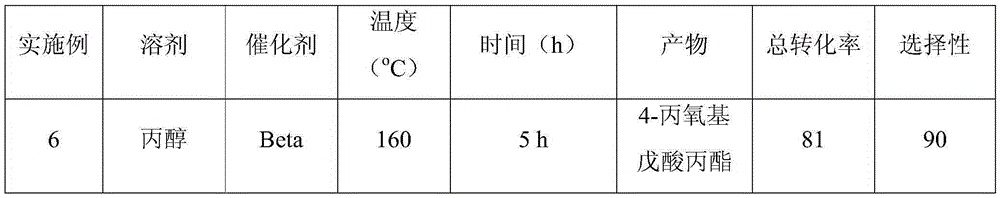
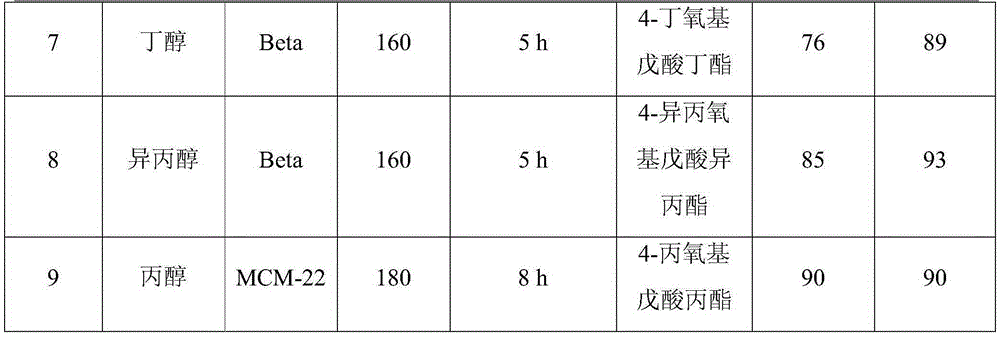
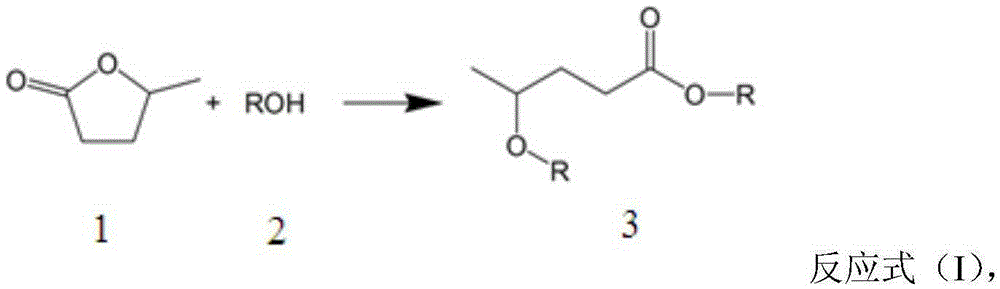
[0018] The molecular sieve Beta was used as a catalyst to prepare 4-ethoxy ethyl valerate (EEP) by ring opening and etherification of γ-valerolactone (GVL). 100mg of catalyst is loaded into the reaction flask, 10g0.25M gamma-valerolactone (GVL) ethanol solution is added, the reaction temperature is 140°C, and the reaction is carried out for 5 hours under self-pressure conditions, and the conversion rate of gamma-valerolactone (GVL) is 76 %, the selectivity of 4-ethoxy ethyl valerate (EEP) was 96%.
[0019] When the molecular sieve materials used are MCM-22, ZSM-5, ZSM-11, USY, Al-SBA-15 molecular sieves, similar technical effects can also be achieved.
Embodiment 2
[0021] The molecular sieve USY was used as a catalyst to prepare 4-ethoxy ethyl valerate (EEP) from γ-valerolactone (GVL) by ring opening and etherification. 100mg of catalyst is loaded into the reaction flask, 10g0.25M gamma-valerolactone (GVL) ethanol solution is added, the reaction temperature is 140°C, and the reaction is carried out for 7 hours under self-pressure conditions, and the conversion rate of gamma-valerolactone (GVL) is 42 %, the selectivity to 4-ethoxy ethyl valerate (EEP) was 63%.
Embodiment 3
[0023] Use molecular sieve Beta as a catalyst to prepare 4-ethoxy ethyl valerate (EEP) by ring-opening and etherification of γ-valerolactone (GVL), put 100mg catalyst in the reaction bottle, add 10g0.25Mγ-valerolactone Esters (GVL) ethanol solution, the reaction temperature is 160 ° C, reacted for 3 hours under self-pressure conditions, the conversion rate of γ-valerolactone (GVL) is 80%, the selection of ethyl 4-ethoxyvalerate (EEP) Sex is 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com