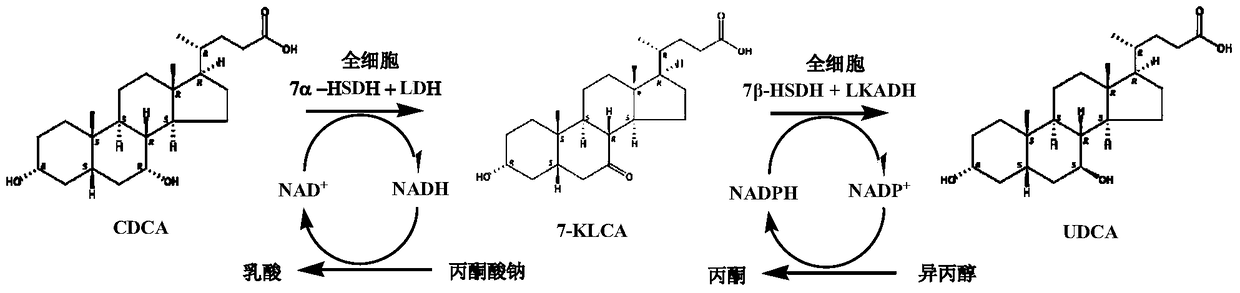
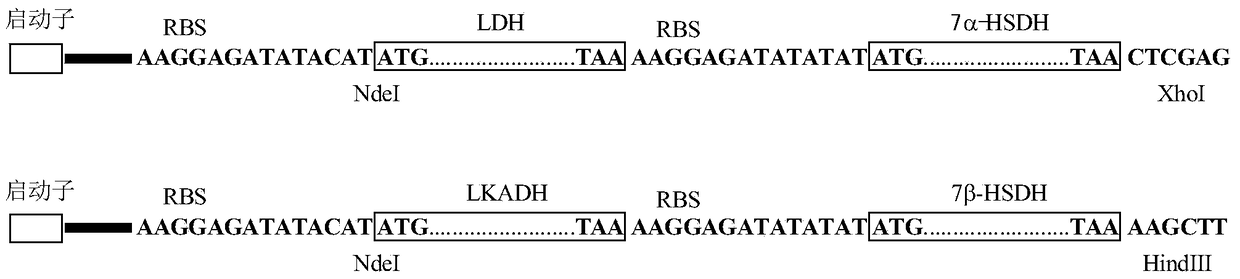
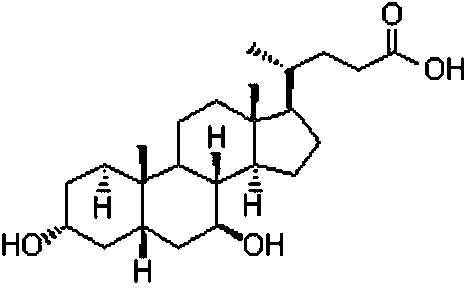
A method for the synthesis of ursodeoxycholic acid from chenodeoxycholic acid with high-efficiency whole-cell catalysis
A technology of ursodeoxycholic acid and cells, applied in the field of bioengineering, can solve problems such as complex process and difficult realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Inoculate Escherichia coli BL21(DE3) containing recombinant DNA pET21a(+)-LDH-7αHSDH into a 250mL Erlenmeyer flask containing 50mL of LB medium (100μg / mL ampicillin), cultivate overnight at 37°C and 300 rpm, and then 10% of the inoculum was transferred into a 2L shake flask containing 400 mL of LB medium (100 μg / mL ampicillin), and cultured at 37° C. and 300 rpm for 2 hours until the OD600 reached 1.0. Add IPTG at a final concentration of 0.2 mM, induce expression at 25°C for 4 hours, and collect cells by centrifugation. The cells were suspended in 20 mL of 100 mM phosphate buffer (pH 8.0), sonicated, and centrifuged to obtain a recombinant mixed crude enzyme solution. The enzyme activities of 7α-HSDH and LDH were 747.0 units / mL and 1083 units / mL, respectively.
Embodiment 2
[0070] E. coli BL21(DE3) containing the recombinant DNA pET21a(+)-LKADH-7βHSDH was subjected to shake flask fermentation using the same method as in Example 1. The induction time was 4 hours. Cells were harvested by centrifugation. The cells were suspended in 20 mL of 100 mM phosphate buffer (pH 8.0), sonicated and centrifuged to obtain a recombinant mixed crude enzyme solution. The enzyme activities of 7β-HSDH and LKADH were 131.8 units / mL and 32.0 units / mL, respectively.
Embodiment 3
[0072] Inoculate a single colony on the Escherichia coli BL21(DE3) plate containing recombinant DNA pET21a(+)-LDH-7αHSDH into two 1L shake flasks filled with 250mL of LB medium (100μg / mL ampicillin), at 37°C and 300 rpm / min for 15 hours with shaking. Combine the seed liquids in the two shake flasks into 500mL and inoculate them into 5L of initial medium containing: 30g / L glycerol, 25g / L potassium dihydrogen phosphate, 10g / L ammonium sulfate , 10g / L magnesium sulfate heptahydrate, 0.4g / L iron sulfate heptahydrate. Recombinant Escherichia coli was cultured with aeration and stirring in a 10L fermenter at a temperature of 30°C and a pH of 7.0. Stirring and aeration were adjusted to control dissolved oxygen at 25%. After the glycerol in the starting medium was exhausted, the induction medium (lactose: 50 g / L, glycerol: 200 g / L) was added to induce the production of the enzyme. The flow rate is 60-250mL / hour, and the maximum flow rate is gradually reached within 3 hours. Temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com