A novel adjuvanted vaccine composition about hpv virus
A vaccine composition and adjuvant technology, applied in the field of vaccine compositions, can solve the problems of aluminum adjuvant safety concerns, nervous system adverse reactions, weak immune effect of the adjuvant, etc., and achieves protection against virus infection, simple preparation method, induction The effect of an efficient immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: Preparation of composite particles of calcium phosphate and polymer PLGA
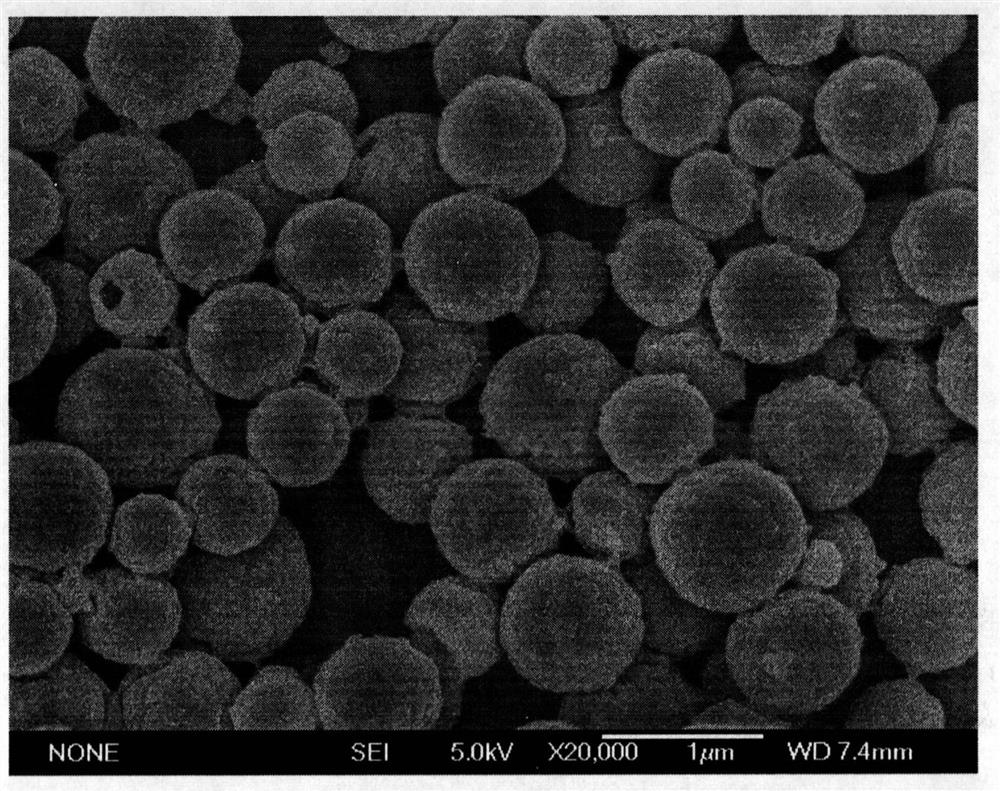
[0046] The PLGA of 100mg is dissolved in the dichloromethane of 20mL as oil phase (O), the 0.1M calcium chloride solution of preparation 0.2mL is as inner water phase (W1), the PVA solution of preparation 1.0% is as outer water phase (W2 ); 0.2mL of the inner water phase (W1) was added to the oil phase (O), and the colostrum W1 / O was prepared by homogeneous emulsification method, and the first emulsion was added to 60mL of the outer water phase to prepare the pre-multiplex emulsion (W1 / O / W2), this pre-multiplex emulsion is repeatedly pressed through the membrane by the method of rapid membrane emulsification to prepare a water-in-oil-in-water complex emulsion (W1 / O / W2) with uniform particle size; then add to this complex emulsion 0.1mL of 0.05M disodium hydrogen phosphate solution, using the solute diffusion of the inner and outer water phases during the solidification process to m...
Embodiment 2
[0047] Embodiment 2: Preparation of composite particles of calcium phosphate and polymer PLGA
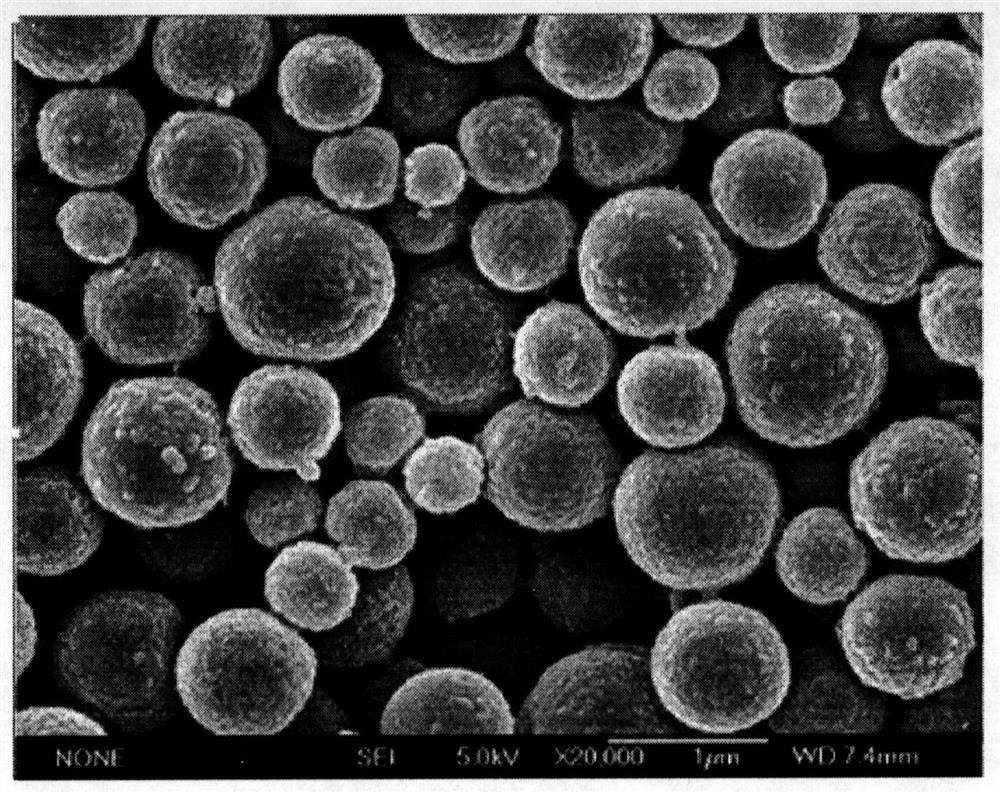
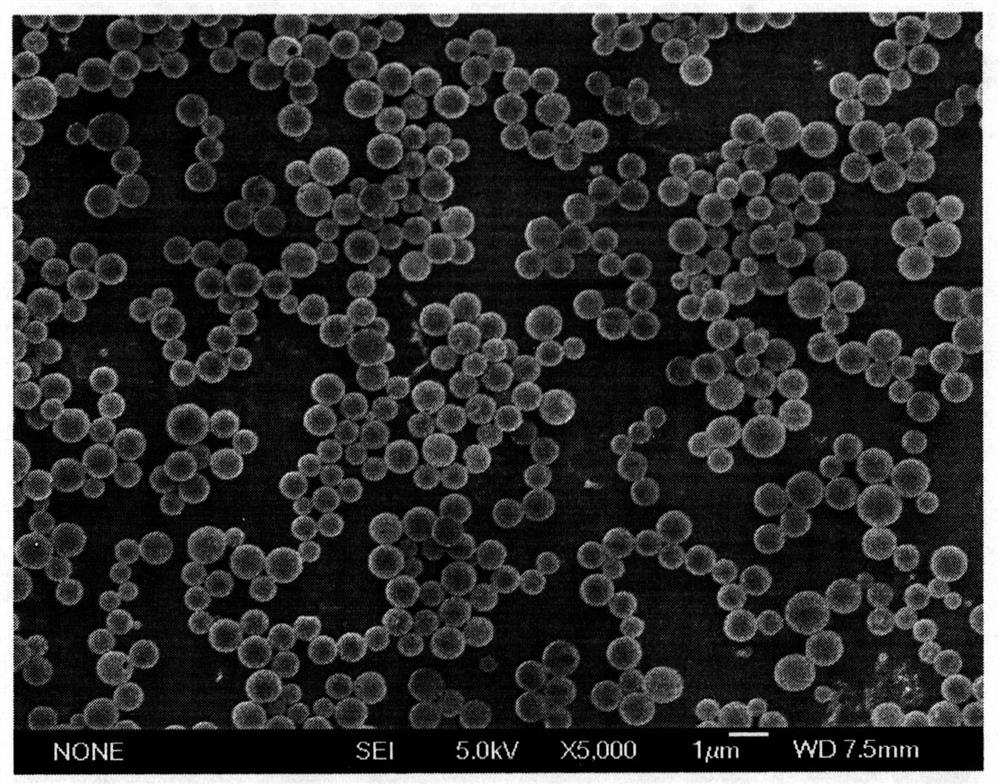
[0048] Dissolve 100mg of PLGA in 20mL of dichloromethane as the oil phase (O), prepare a 1.0% PVA solution as the external water phase (W2), add the oil phase (O) to 50mL of the external water phase (W2) , prepare the pre-emulsion (W2 / O) of oil-in-water type, this pre-multiple emulsion adopts the method for rapid film emulsification to repeatedly press through the membrane to prepare the water-in-oil-in-water double emulsion (W1 / O / O) with uniform particle size W2); solidified for 3h, centrifuged and washed, freeze-dried to obtain dry PLGA microspheres, and the SEM photo of PLGA is as follows figure 2 shown. Then suspend the prepared PLGA microspheres in 10mL ultrapure water to obtain PLGA microsphere suspension, prepare 0.2mL of 0.2M calcium chloride solution and 0.1M disodium hydrogen phosphate solution, and first mix 0.2mL of 0.2M Calcium chloride solution was added to 10mL of ...
Embodiment 3
[0049] Embodiment 3: the preparation of the composite particle of calcium phosphate and polymer PLGA
[0050] The PLGA of 100mg is dissolved in the dichloromethane of 20mL as oil phase (O), the 0.6M calcium chloride solution of preparation 0.2mL is as inner water phase (W1), the PVA solution of preparation 1.0% is as outer water phase (W2 ); 0.2mL of the inner water phase (W1) was added to the oil phase (O), and the colostrum W1 / O was prepared by homogeneous emulsification method, and the first emulsion was added to 60mL of the outer water phase to prepare the pre-multiplex emulsion (W1 / O / W2), this pre-multiplex emulsion is repeatedly pressed through the membrane by the method of rapid membrane emulsification to prepare a water-in-oil-in-water complex emulsion (W1 / O / W2) with uniform particle size; then add to this complex emulsion 0.1mL of 0.3M disodium hydrogen phosphate solution, using the solute diffusion of the inner and outer water phases during the solidification proces...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com