Zinc phthalocyanine hydriodate photosensitizer, as well as preparation method and application thereof
A technology of zinc phthalocyanine hydroiodide and photosensitizer, which is applied in the field of photosensitizers, can solve problems such as complex organic reactions and separation and purification processes, and achieve good application prospects, low dark toxicity, and high singlet oxygen quantum yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0052] The synthesis of embodiment 12,9,16,23-four-((4-aminomethyl) phenoxy group) zinc phthalocyanine hydroiodide
[0053] (1) Synthesis of 4-((4-aminomethyl)phenoxy)phthalonitrile
[0054] The reaction formula is as follows:
[0055]
[0056] Under nitrogen protection, 4-nitrophthalonitrile, p-hydroxybenzylamine and K 2 CO 3 Added into DMF at a molar ratio of 1:1:2, then added 10mL of dichloromethane as a solvent, and reacted at 60°C for 6h to obtain intermediate 3;
[0057] (2) Synthesis of 4-(4-((tritylamino)methyl)phenoxy)phthalonitrile
[0058] The reaction formula is as follows:
[0059]
[0060] Under the protection of nitrogen, triphenylchloromethane was dissolved in dichloromethane, and slowly dropped into the dichloromethane solution of intermediate 3 and potassium carbonate with a constant pressure dropping funnel, intermediate 3 was mixed with triphenylchloromethane and potassium carbonate The molar ratio is 1:1.2:2.5, titrate for about 5 hours, and the...
Embodiment 2
[0074] The synthetic method of 2,9,16,23-tetra-((4-aminomethyl) phenoxy) zinc phthalocyanine hydroiodide, the steps are as follows:
[0075] (1) Synthesis of 4-((4-aminomethyl)phenoxy)phthalonitrile
[0076] Under nitrogen protection, 4-nitrophthalonitrile, p-hydroxybenzylamine and LiOH were added to DMF at a molar ratio of 1:1.5:2, and then 10 mL of dichloromethane was added as a solvent to react at 80°C for 6 hours to obtain intermediate 3 ;
[0077] (2) Synthesis of 4-(4-((tritylamino)methyl)phenoxy)phthalonitrile
[0078] Under the protection of nitrogen, triphenylchloromethane was dissolved in dichloromethane, and the constant pressure dropping funnel was slowly dropped into the dichloromethane solution of intermediate 3 and LiOH. The molar ratio of intermediate 3 to triphenylchloromethane and LiOH was 1:1.5:2, titrate for about 5 hours, and then react at room temperature for 4 hours to obtain intermediate 4;
[0079] (3) Synthesis of 2,9,16,23-tetra-((4-(((trityl)amin...
Embodiment 3
[0086] The synthesis of 2,9,16,23-tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine hydrochloride (II) is as follows:
[0087]
[0088] (1) According to the steps (1) to (4) of Example 1, 2,9,16,23-tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine was obtained;
[0089] (2) Synthesis of 2,9,16,23-tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine hydrochloride
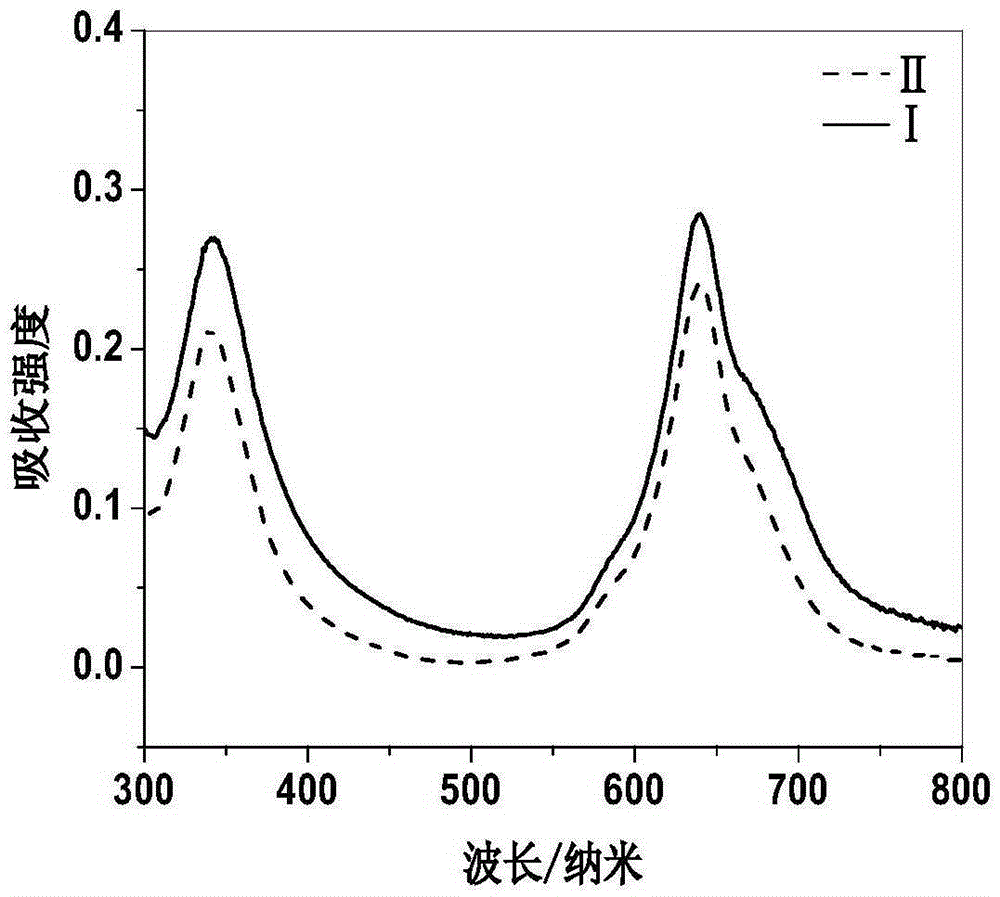
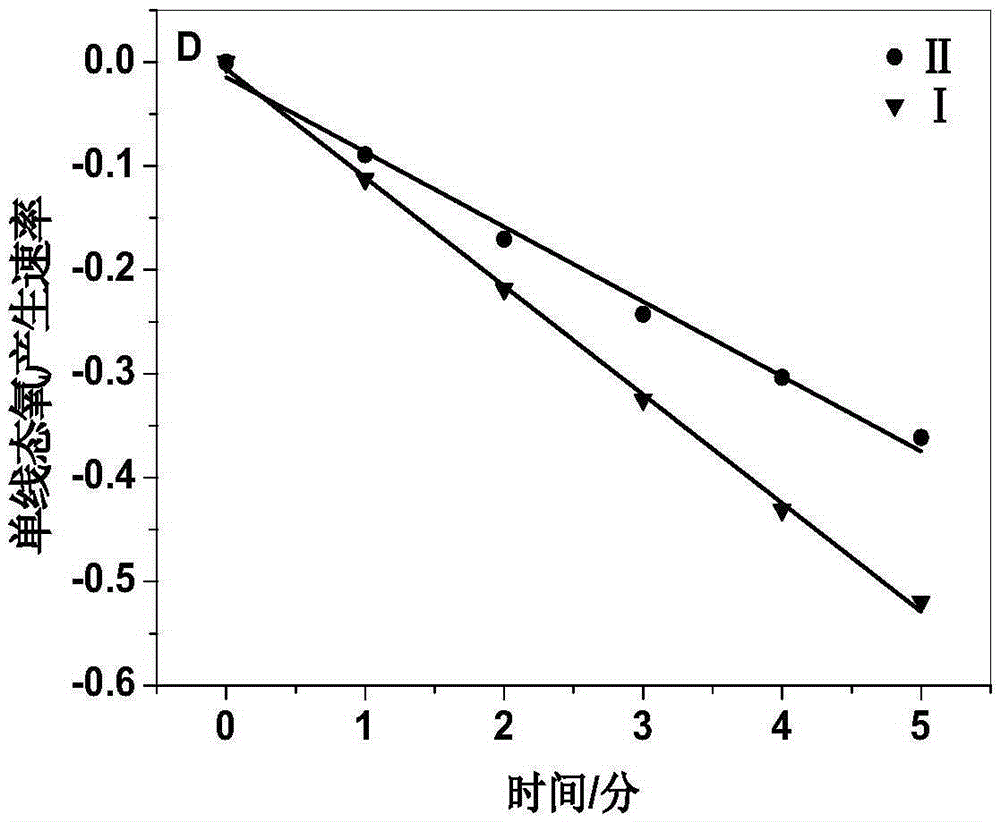
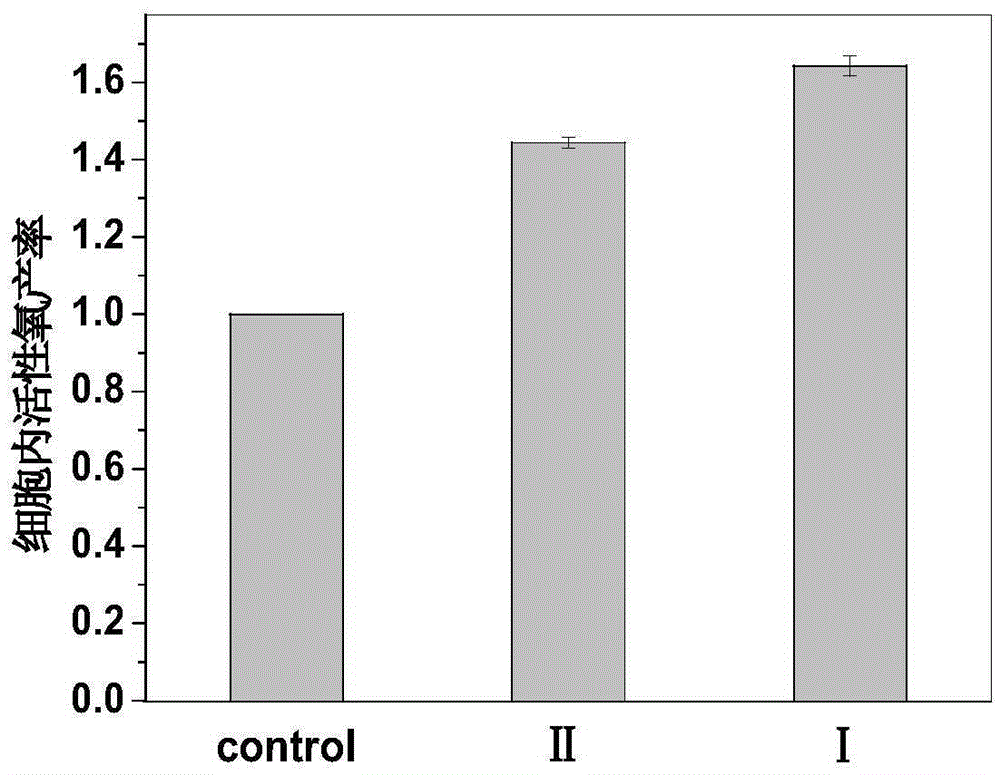
[0090] Suspend 2,9,16,23-tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine 6 in distilled water, add 30 equivalents of 5% hydrochloric acid, and stir at room temperature until 2,9,16 , 23-Tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine 6 was completely dissolved. The reaction solution was concentrated, poured into 50 mL of acetone, and blue-green flocculent solids were precipitated. After filtration, 2,9,16,23-tetrakis-((4-aminomethyl)phenoxy)zinc phthalocyanine hydrochloride II was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com