A kind of mutant type sus scrofa pig-derived trypsin and its coding gene as well as its acquisition method and application
A trypsin gene and trypsin technology, applied in the field of molecular enzymology and biology, can solve the problems of complicated analysis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
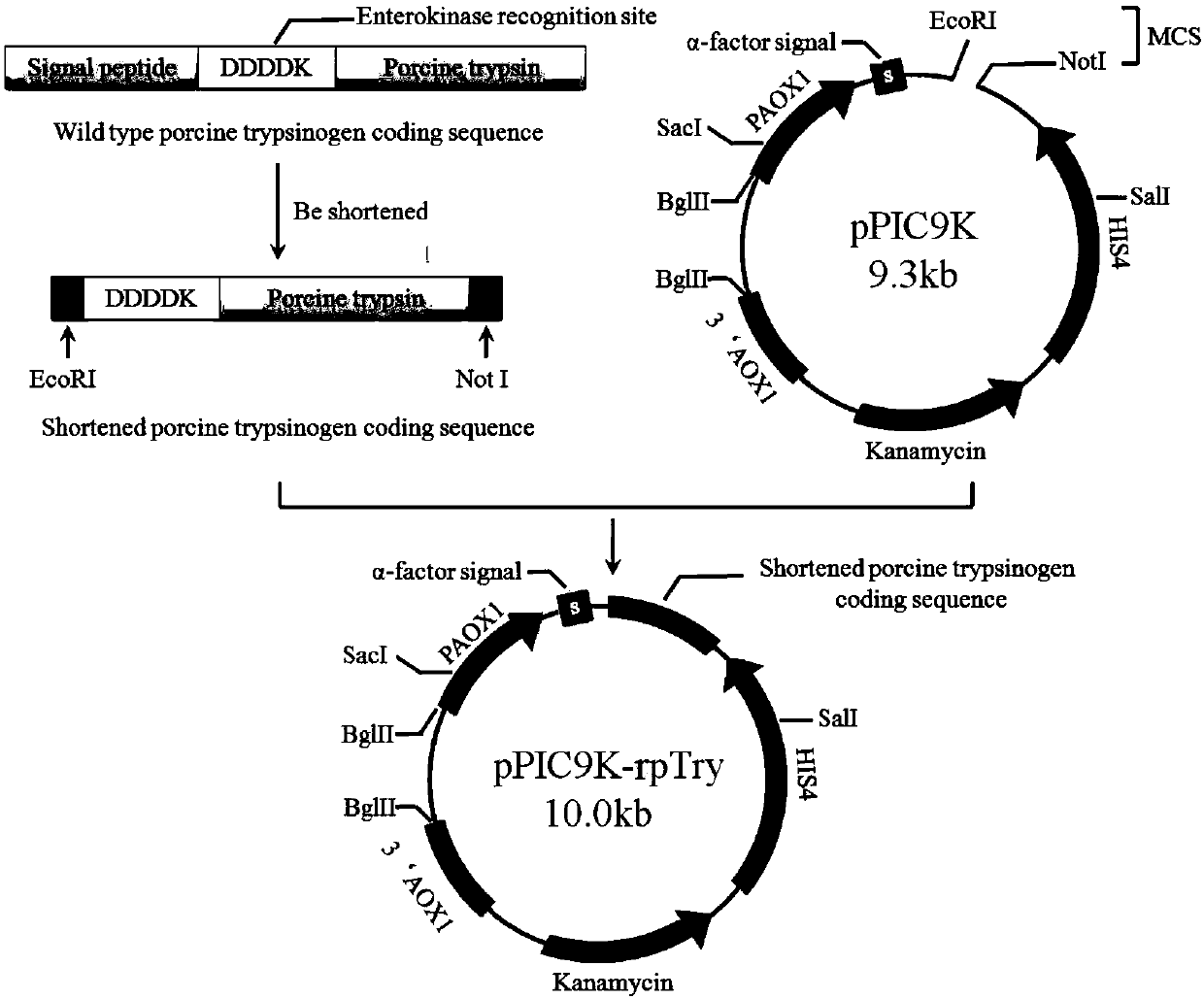
[0064] (1) Construction of wild-type Sus scrofa pig-derived trypsinogen expression vector
[0065] The constructed wild-type recombinant porcine trypsinogen expression vector pPIC9K-rpTry vector was synthesized from the whole gene of Beijing Jinweizhi Co., Ltd., as shown in figure 1 shown. Among them, the first 20 amino acids of the first 25 amino acids of the N-terminal zymogen activating peptide of the original gene were removed, and the remaining sequence of the N-terminal activating peptide was the enterokinase recognition site (DDDDK). The shortened Sus scrofa porcine zymogen sequence was digested with EcoRI and NotI and inserted into the corresponding single cloning site of pPIC9K.
[0066] (2) Quick-Change PCR technology to obtain mutant vector
[0067] According to the primer design principles of the TransGen Fast Mutagenesis System Kit, we designed mutation primers for the mutation N84S, as shown in Table 1-1. The polymerase for the PCR reaction was Phusion High-fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com