A class of ferrocene flame retardant containing phosphorus and nitrogen elements and its preparation method and use
A technology of nitrogen element and ferrocene salt, which is applied in functional metallocene compounds and its application fields, can solve the problems affecting resin performance and residue, achieve good flame retardant effect, small addition amount, and facilitate industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 flame retardant ( n 6 -4-(N-(1-p-hydroxyphenyl-1-DOPO)methyl)aminophenoxybenzene)( n 5 -Synthesis of iron hexafluorophosphate FCOPH
[0038] The synthesis process is shown in the following formula:
[0039]
[0040] In a 250ml round bottom flask add ( n 6 -chlorobenzene)( η5 -Cyclopentadiene) iron hexafluorophosphate (Fc-Cl) 20g (0.026mol), 4-aminophenol 7g (0.032mol), potassium carbonate 10g (0.036mmol), N,N-dimethylformamide 120ml , react in the dark at room temperature (25°C), and monitor the reaction with a thin-layer chromatography spot plate until ( n 6 -chlorobenzene)( n 5 -cyclopentadiene) iron hexafluorophosphate reacts completely. The reactant was poured into cold water, a yellow solid was precipitated, left to stand, filtered under reduced pressure, and the filter cake was rinsed with ethanol three times to obtain the target compound ( n 6 -aminodiphenyl ether) ( n 5 -cyclopentadiene) iron hexafluorophosphate, yellow so...
Embodiment 2
[0043] Embodiment 2 flame retardant ( n 6 -4-(N-(1-phenyl-1-DOPO)methyl)aminophenoxybenzene)( n 5 -Synthesis of iron hexafluorophosphate (FCOP)
[0044] The synthesis process is shown in the following formula:
[0045]
[0046] Add ( n 6 – aminodiphenyl ether) ( n 5 -Cyclopentadiene)iron hexafluorophosphate (Fc-NH 2 ) 20g (0.044mol), 4.7g (0.044mol) benzaldehyde and 100ml DMF, protected from light, stirred at 30°C for 5 hours, then added 9.6g (0.044mol) DOPO, monitored the reaction by TLC, and reacted in 13 hours End. After the reaction was completed, the reaction solution was poured into cold water, a yellow solid was precipitated, left standing, and filtered to obtain the target product (yield: 82%).
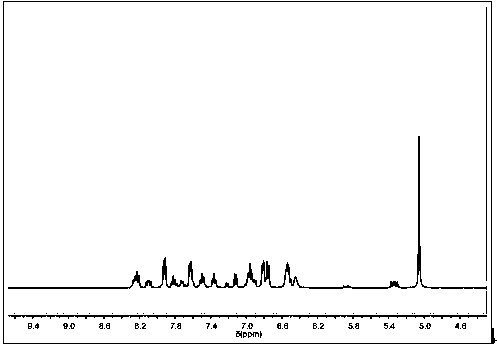
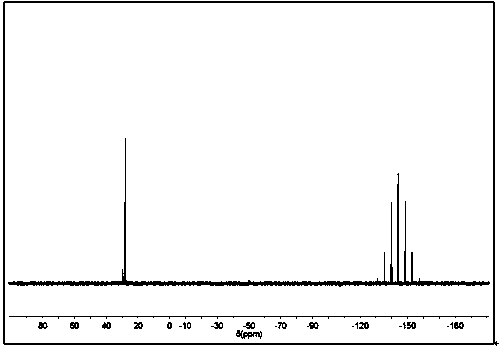
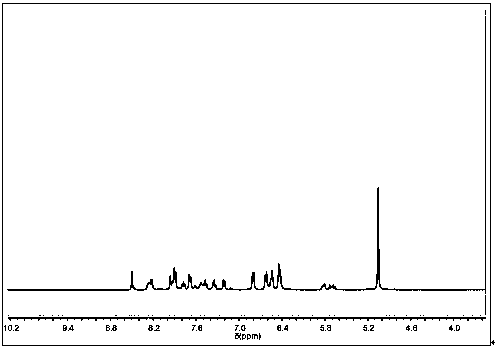
[0047] (KBr) ν max (cm−1): 842 (PF 6 - ), 929 (P-O-C), 1237 (P=O), 1456, 1477, 1503, 1607 (Ar ring), 3090, (Ar-H); 1 H-NMR (400 MHz, DMSO- d 6 ) δ: 5.08 (s, 5H, CP-H), 5.15 (m, 1H, P-C-H), 6.10 (m, 3H, Ar-H), 6.25 (t, 2H, Ar-H), 6.65 (m, 1H , Ar-H), 6.8...
Embodiment 3
[0048] Embodiment 3 flame retardant ( n 6 -4-(1-(4-Hydroxyanilino)-1-DOPO)methylbiphenyl) ( n 5 -Synthesis of iron hexafluorophosphate (FCPH)
[0049] The synthesis process is shown in the following formula:
[0050]
[0051] Add 50g (0.13mol) ( n 6 -chlorobenzene)( n 5 -cyclopentadiene)iron hexafluorophosphate, 22.5g (0.15mol) 4-formylphenylboronic acid, 2 mol∙L‾ 1 Potassium carbonate aqueous solution 100ml, tetrahydrofuran 200ml, tetrakistriphenylphosphine palladium 0.3g, under the protection of nitrogen, heated to reflux under the condition of avoiding light. The reaction was monitored by thin-layer chromatography, and the reaction was completed within 12 hours. After cooling, filter to remove insoluble impurities, wash the organic phase three times with water, keep the organic phase, concentrate, add methyl tert-butyl ether to the concentrated solvent, a large amount of yellow solids are precipitated, filter to obtain the product, ( n 6 –4-Formyl bipheny...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com