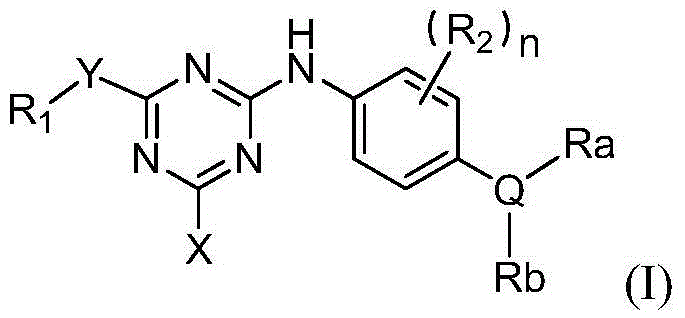
Triazine epidermal growth factor acceptor inhibitor and application thereof
A technology of isomers and solvates, applied in the field of cancer treatment drugs, can solve the problems of drug resistance and lack of selectivity in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1-methylindol-4-yl) -1,3,5-Triazine-2-amino)phenyl)acrylamide
[0109]
[0110] Step a Synthesis of 4-chloro-N-(4-fluoro-2-methoxy-5-nitrophenyl)-1,3,5-triazin-2-amine
[0111]
[0112] In a 100ml reaction flask, sequentially add 4-fluoro-2-methoxy-5-nitroaniline (4.0g, 21.5mmol), DIPEA (8.32g, 64.5mmol) and 60ml tetrahydrofuran, dissolve and cool to 0-2 ℃, add 2,4-dichloro-1,3,5-triazine (3.14g, 21.08mmol) in batches, after the addition is complete, continue the reaction for 5h, stop the reaction, add 60ml of water, stir, filter, and wash the filter cake with water , and dried to obtain the title compound, which was directly used in the next step.
[0113] Step bN-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methylindol-4-yl)-1,3,5-triazin-2-amine synthesis
[0114]
[0115] In a 30ml microwave reaction vial, add 1-methylindole-4-boronate (800mg, 3.11mmol) and 4-chloro-N-(4-fluoro-2-methoxy-5...
Embodiment 2
[0127] Example 2N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1-methylindol-2yl)- 1,3,5-Triazine-2-amino)phenyl)acrylamide
[0128]
[0129] Step aN-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methylindol-2-yl)-1,3,5-triazin-2-amine synthesis
[0130]
[0131] 4-chloro-N-(4-fluoro-2-methoxy-5-nitrophenyl)-1,3,5-triazin-2-amine and 1-methyl Using indole-2-boronic acid as starting material, the title compound was prepared according to the method in step b of Example 1.
[0132]Step b2 N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(1-methylindol-2yl)-1 , Synthesis of 3,5-triazine-2-amino)phenyl)acrylamide
[0133] N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(1-methylindol-2-yl)-1,3,5-triazine- Using 2-amine, N,N,N'-trimethylethylenediamine and allyl acid chloride as raw materials, the title compound was prepared according to the methods in step c, step d and step e of Example 1.
[0134] 1 H-NMR (300MHz, DMSO-d 6 )δ:2.50-2.73(8H,m),3.09-3.21(5H,m...
Embodiment 3
[0136] Example 3N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-(4-(3-(1-methylcarbamoylbenzene base))-1,3,5-triazine-2-amino)phenyl)acrylamide
[0137]
[0138] With 4-fluoro-2-methoxy-5-nitroaniline, 2,4-dichloro-1,3,5-triazine, 3-(methylcarbamoyl)phenylboronate, N,N, N'-trimethylethylenediamine and allyl acid chloride were used as raw materials, and the title compound was prepared according to the method of Example 1.
[0139] 1 H-NMR (500MHz, DMSO-d 6 )δ:2.25(6H,d),2.37-2.41(m,2H),2.72(3H,s),2.80(3H,s),2.92(2H,m),3.81(3H,s),5.75-5.77 (1H,d),6.24-6.(1H,d),6.42-6.48(1H,m),7.02(1H,s),7.62(1H,s),8.02-8.03(1H,d),8.27( 1H,s), 8.59(2H,s), 8.77(1H,s), 8.83, (1H,s), 9.27(1H,s), 10.11(1H,s).
[0140] ESI-Msm / z: 505.3 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com