Application of GW3965 as PARP1 inhibitor
An inhibitor, trifluoromethyl technology, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Detection by Cell-Free PARP1 Enzymatic Reaction System
[0028] A PARP1 activity detection kit is used to construct a cell-free PARP1 enzymatic reaction system, and the reaction system can activate the activity of PARP1 enzyme in an in vitro environment.
[0029] The PARP1 activity detection kit used in the examples was purchased from Trevigen, produced in the United States, with a model number of 4676-096-K.
[0030] PARP1 activity detection kit includes buffer, PARP1 recombinant protein, NAD + , single-strand break DNA, recombinant histone 1 (HistoneH1).
[0031] Configure the cell-free PARP1 enzymatic reaction system according to the PARP1 activity detection kit, the specific operation steps are as follows: add 50ng PARP1 recombinant protein (PARP1protein) to the buffer solution, the final concentration is 10mmol / LNAD + , The final concentration is 20mg / mL after single-strand break DNA. The final concentrations of the various components that make up the ...
Embodiment 2
[0038] Embodiment 2 utilizes in vitro cell experiment to detect
[0039] In the in vitro cell experiment, HepG2 (derived from American Type Culture Collection) cells were selected as experimental cells. The inventors treated HepG2 cells with different concentrations of 22(R)-hydroxycholesterol (abbreviated as 22(R)-HC), T09013171 and GW3965. For treatment methods, see HuangD, YangC, WangY, LiaoY, & HuangK. PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl)ation of PPARgammaincardiacfibroblasts. Cardiovascularresearch, 2009, 81(1): 98-107.
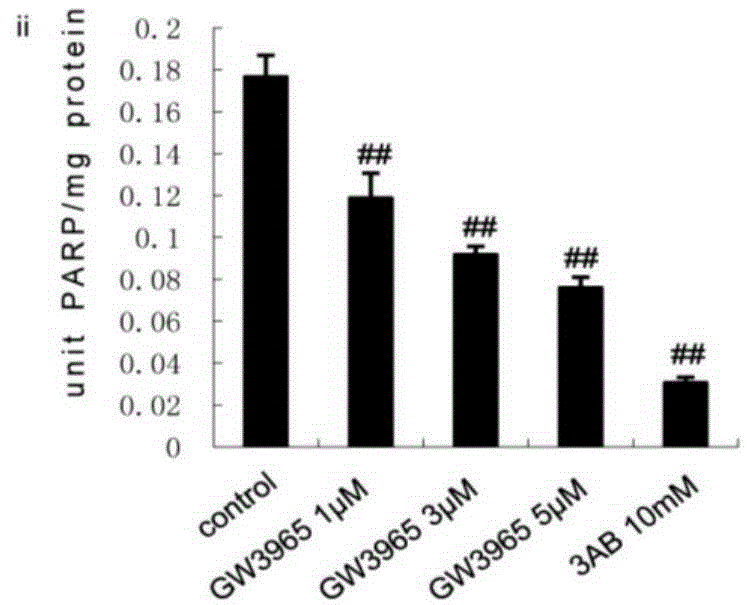
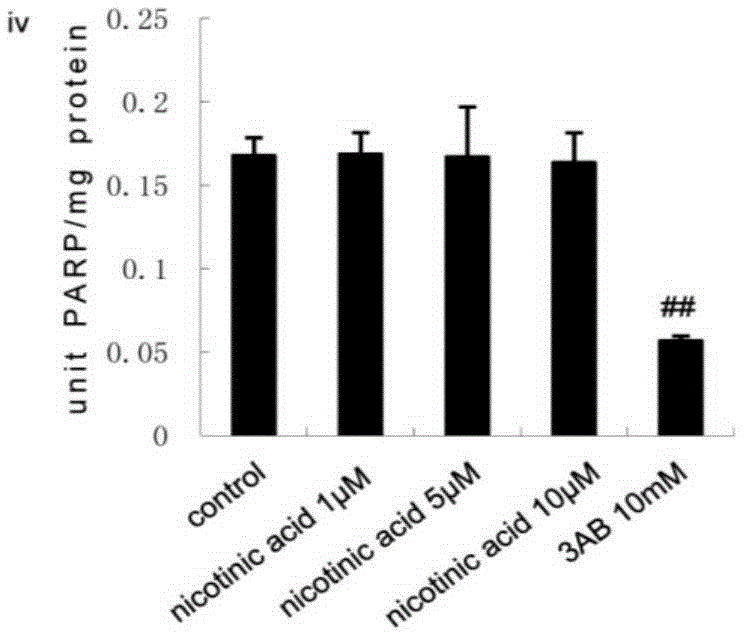
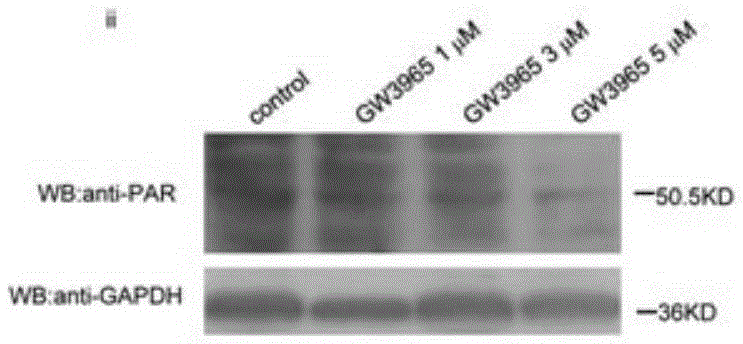
[0040] HepG2 cells were stimulated with GW3965 (ii, 1 μmol / L, 3 μmol / L, 5 μmol / L) or niacin (iv, 1 μmol / L, 5 μmol / L, 10 μmol / L) for 24 hours, and the concentrations in the above brackets were final concentration.
[0041] PARP1 activity was detected using a PARP1 activity detection kit (purchased from Trevigen, produced in the United States, model number 4676-096-K). For specific methods, refer to the instructions of the k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com