Method for extracting and separating nepeta glucoside, homoplantaginin and apigenin glucoside from elsholtzia splendens
A technology of pseudonepetaloside and apigenin glycoside is applied in the field of biological resource utilization of natural products, and can solve the problems of not informing and extracting flavonoid glycosides and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
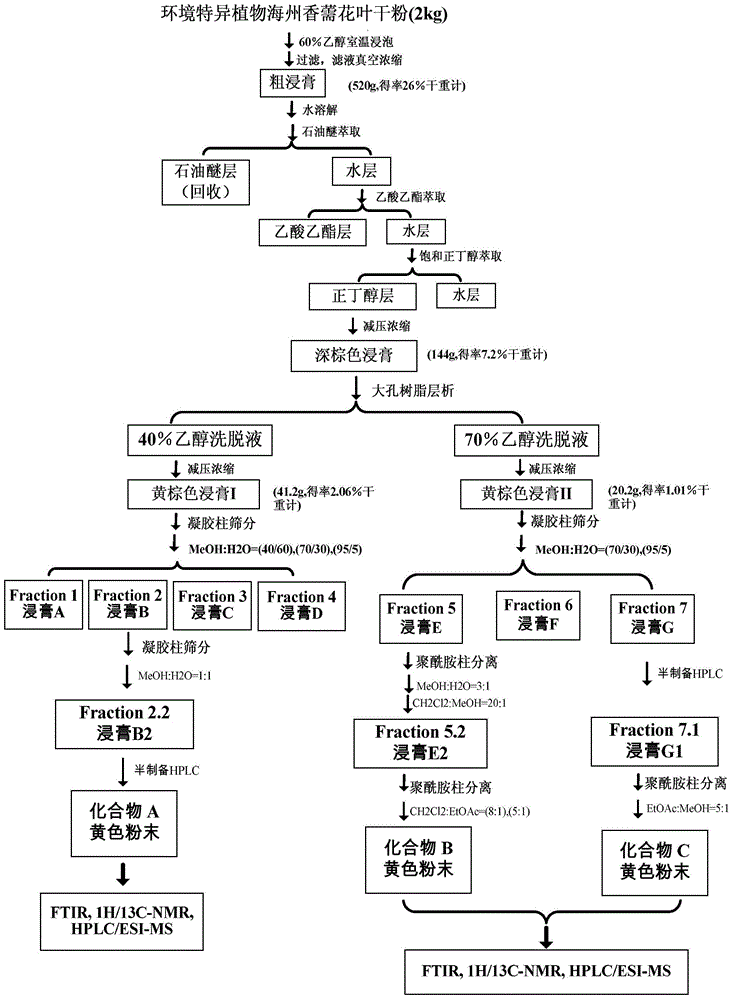
[0055] Embodiment 1, ethanol extraction / extraction, macroporous resin separation, gel column sieving of the flower and leaf of Rhizoma haizhou of the present invention:
[0056] (1) Alcohol extraction and liquid-liquid extraction (ie, leaching and extraction):
[0057] Air-dry the flower branch and leaves of Cyperus haizhou harvested in full flowering stage (to water content ≤ 0.5%, weight %) and then pulverize (to pass through a 60-mesh sieve). Take the above-mentioned 2kg sample, place it in a 50L plastic bucket, add 30L of 60% (V / V) ethanol, stir evenly and soak for at least 30 days, and filter with gauze to obtain the filtrate. Repeat the above leaching process twice for the remaining residue obtained by filtration. The filtrates obtained by leaching three times were combined; concentrated in vacuo under the condition of heating in a water bath at 40° C. to obtain 520 g of crude extract; the yield was 26.00% (by dry weight).
[0058] The medicinal extract of above-mentio...
Embodiment 2
[0084] Example 2. Separation and purification of nepetalin, homopsyllogenin and apigenin-7-O-β-D-glucoside in the Herba pilosula of the present invention
[0085] (1) Separation and purification of nepetalin
[0086] ① Gel column re-screening: 50g of Sephadex TM Dissolve LH-20 in 200ml of methanol, stir to remove air bubbles, and pack into a column under normal pressure. 0.69 g of extract B (Fraction 2) obtained in Example 1 was dissolved with 5 ml of 50% methanol / water, and slowly loaded onto the top of the gel column. With the volume ratio of methanol:water = 1:1 as the development system, carry out normal pressure column elution, a total of 500ml of eluent is required, collect the effluent from each bottle (collect 1 tube per 50ml), track the plate, and use ultraviolet light Dark red at 365nm, mass concentration 3% FeCl 3 The solution is dark green and the two indicators shall prevail, and the R f = 0.5 (the ratio shift value of the spot on the polyamide film) and the t...
Embodiment 3
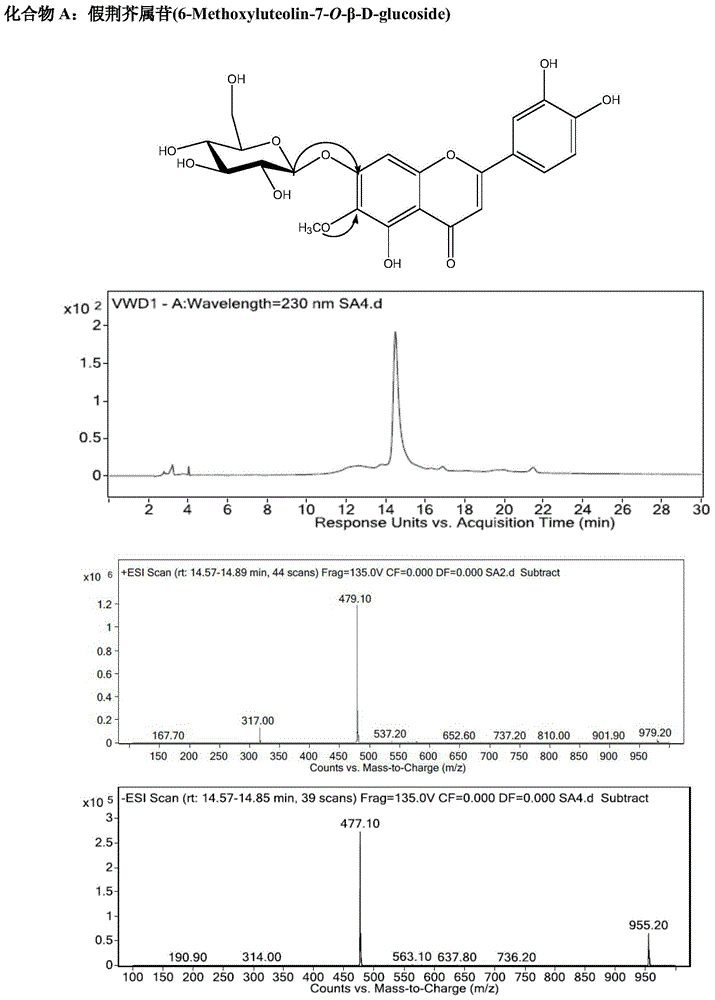
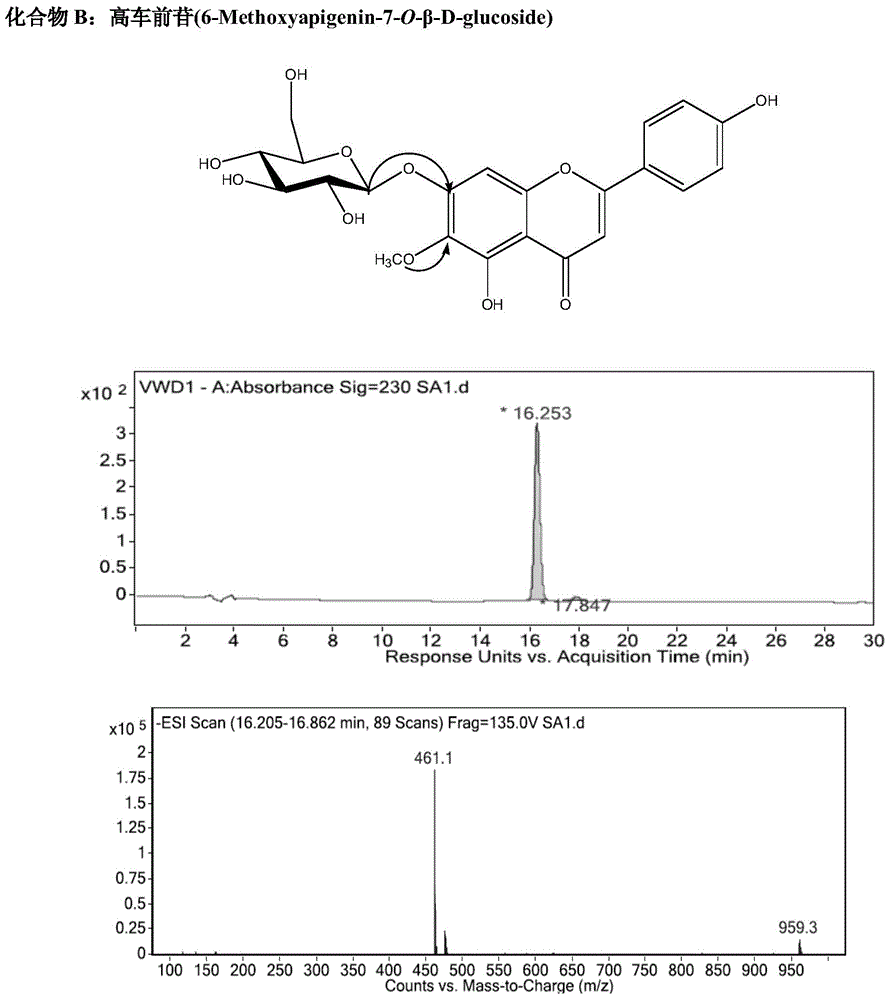
[0097] Example 3. Identification of chemical structures of nepetalin, homopsyllogenin, and apigenin-7-O-β-D-glycoside in Cyperus haizhouensis of the present invention
[0098] The purified product of the yellow powder obtained in Example 2 (compound A—nepetalin, B—homopsyllogenin, C—apigenin-7-O-β-D-glucoside), respectively, with Deuterated DMSO was dissolved, and H-NMR, C-NMR, and HPLC / ESI-MS mass spectra were determined for structure identification. Data are as follows:
[0099] Nepetatin (purity 95%): yellow powder. UV absorption maximum peak λ max (nm) (methanol): 270,344. IR(KBr,cm -1 ): 3384 (-OH), 1357 (benzene ring-OH), 1267 (benzene ring-OH), 2925 (glycoside saturated C-H), 1650 (C=O), 1602 (benzene ring C=C), 1498 (benzene Ring C-H), 1451, 1400, 1124, 1069, 1010, 910, 837, 772. The characteristic peaks of proton NMR spectrum and carbon spectrum belong to: 1 HNMR (500MHz, DMSO-d 6 )δ(ppm):3.90(3H,s,6-OCH 3 ),6.72(1H,s,H-3),6.87(1H,s,H-8),6.89(1H,d,J=8.8Hz,H-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com