Contrast medium formulation and related preparation method
An advantageous composition technology, applied in preparations for in vivo tests, medical preparations containing active ingredients, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
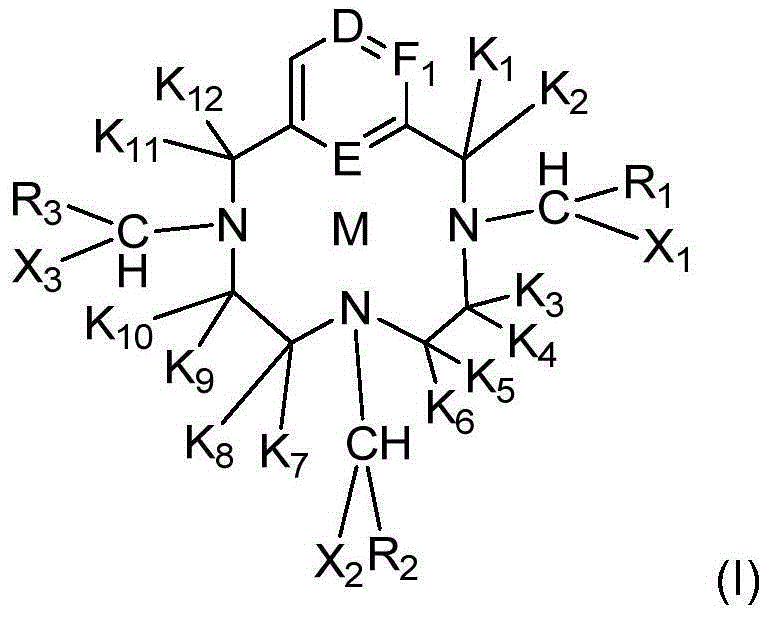
[0059] In particular, the concentration of the above-mentioned complex of formula (I) according to the composition of the invention is between 0.001 and 1.5 mol.l -1 Between, preferably between 0.2 and 0.7mol.l -1 Between, more preferably between 0.3 and 0.6mol.l -1 between.
[0060] The complex of formula (I) is determined by methods known to those skilled in the art. In particular, it can be determined after mineralization and determination of all paramagnetic metals present in the composition. In the case of determining the total gadolinium present in solution, the determination is carried out by optical emission spectrometry (also known as ICP-AES or ICP atomic emission spectrometry).
[0061] The content of the complex of formula (I) allows the composition to have optimum contrast capability while having a satisfactory viscosity. Indeed, the above-mentioned complexes of formula (I) have less than 0.01 mol.l -1 , the level of performance as a contrast product is less ...
Embodiment 1
[0109] Embodiment 1: Embodiment according to the manufacturing method of the present invention
[0110] The method for making the composition is carried out according to the following steps:
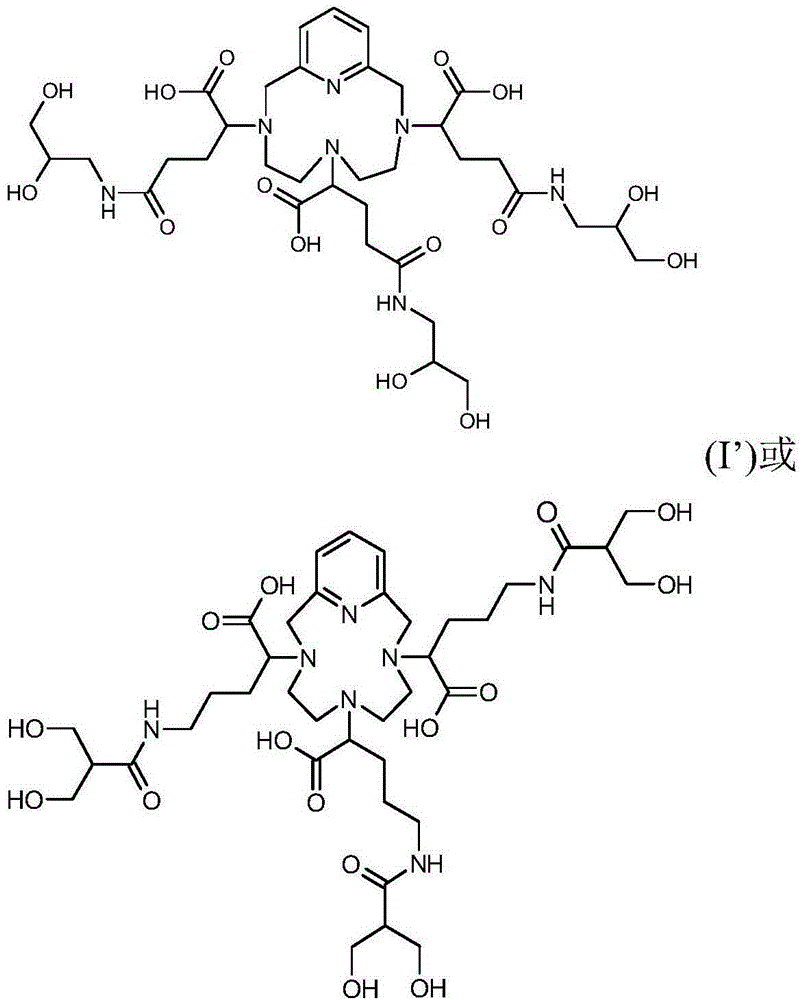
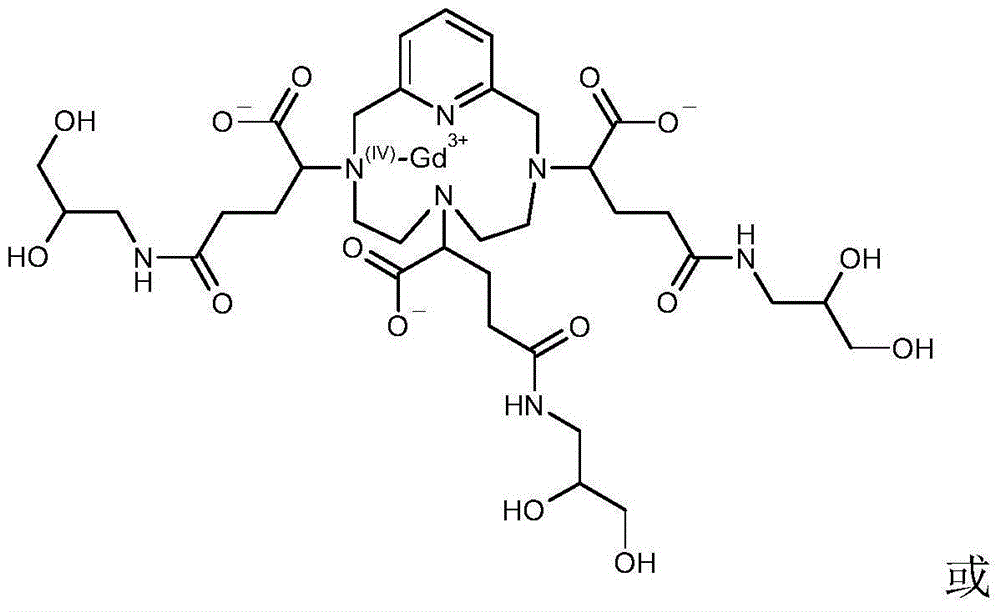
[0111] a) 485.1 g (ie 0.5 M) of the chelating ligand of formula (I′) in the form of an odorless white powder by heating the vessel to a temperature of 50° C. and stirring the solution vigorously until the complex is completely dissolved in water with gadolinium ions (Gd 3+ ) The complex between is dissolved in water (1 liter of appropriate amount). The solution was then cooled to about 30°C.
[0112]
[0113] b) Add 1.011 g (ie relative to the ratio of the complex added in step a), 0.5% mol / mol) of DOTA (Simafex, France) to the solution obtained in step a).
[0114] c) Add 0.368 g (ie relative to the ratio of the complex compound added in step a), 0.5% mol / mol) of calcium chloride (CaCl) to the solution obtained in step b). 2 ,2H 2 O) (Merck).
[0115] c') If necessary, the pH o...
Embodiment 2
[0118] Example 2: Example of a composition according to the invention and results from studies of said composition.
[0119] By means of the method of Example 1, the following preparations are obtained:
[0120]
[0121] *Measured by colorimetry using xylenol orange
[0122] Stability under accelerated conditions and long-term stability studies
[0123] The expression "study of stability under accelerated conditions" is intended to mean a study carried out at 40°C over the course of 6 months and the expression "study of long-term stability" is intended to mean a study of 25°C over the course of 36 months Studies performed at °C (ICH conditions).
[0124] Two main entities present in the composition were measured over time.
[0125]
[0126] *Av.s = before sterilization
[0127] **Ap.s = after sterilization
[0128] ***NA = not analyzed
[0129] Free gadolinium was neither detected nor quantified in the composition. The amount of DOTA-calcium complex decreased sign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com