Crystal form b of abt-888 and its preparation method and application
A technology of ABT-888 and crystal form, applied in organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problems of high operation requirements, poor solubility, complicated preparation process, etc., and achieve low cost , Solubility improvement, simple operation effect
Active Publication Date: 2018-02-16
CRYSTAL PHARMATECH CO LTD
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Among the two crystal forms, the solubility of crystal form 1 is relatively good, but crystal form 1 needs to react with the acid or diacid salt of ABT-888 with a base, and the crystal form 1 mixed with one or more than one solvent can be made by deprotonation reaction. ABT-888 is obtained by crystallization or recrystallization in the form of solid, semi-solid, wax or oil. The preparation process is relatively complicated and the requirements for operation are also high.
Although the preparation of crystal form 2 is relatively simple, it can be obtained by completely dissolving ABT-888 in methanol, then concentrating at 35°C, and drying to constant weight, but its solubility is poor
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0030] Dissolve 21.8 mg of the free base of the compound of formula I in 1.5 mL of methanol and volatilize at room temperature (25±1° C.) to obtain solid crystals, which are labeled as sample 1.
Embodiment 2
[0032] Dissolve 20.7 mg of the free base of the compound of formula I in 1.0 mL of methanol and volatilize at room temperature (25±1° C.) to obtain solid crystals, which are labeled as sample 2.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
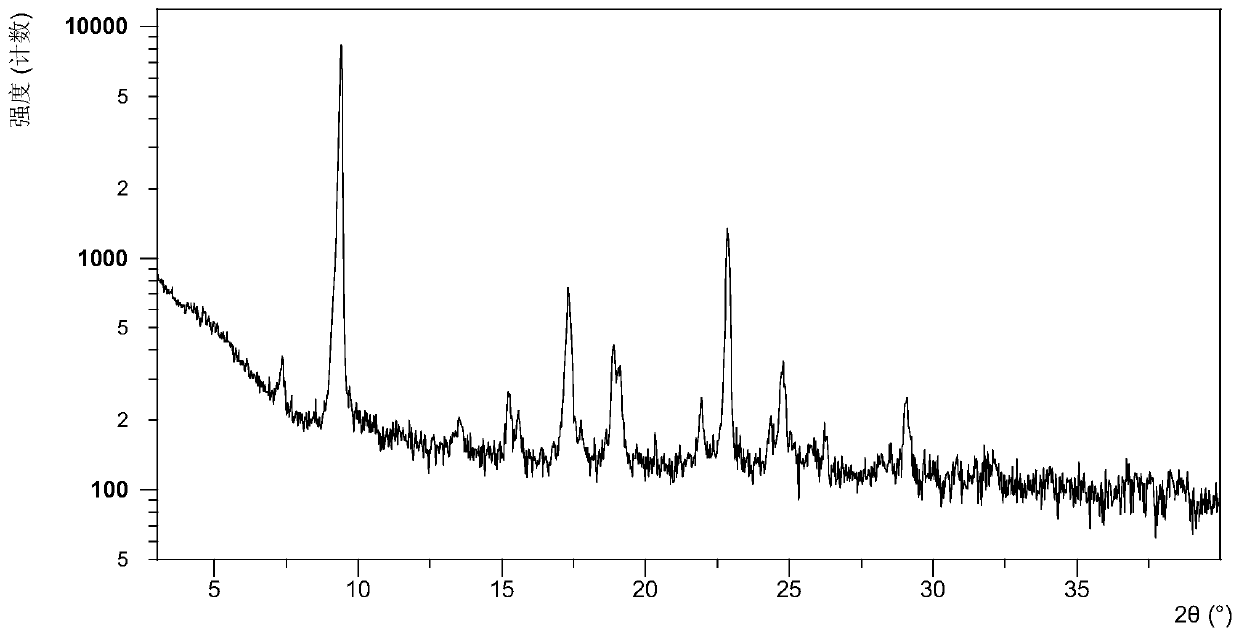
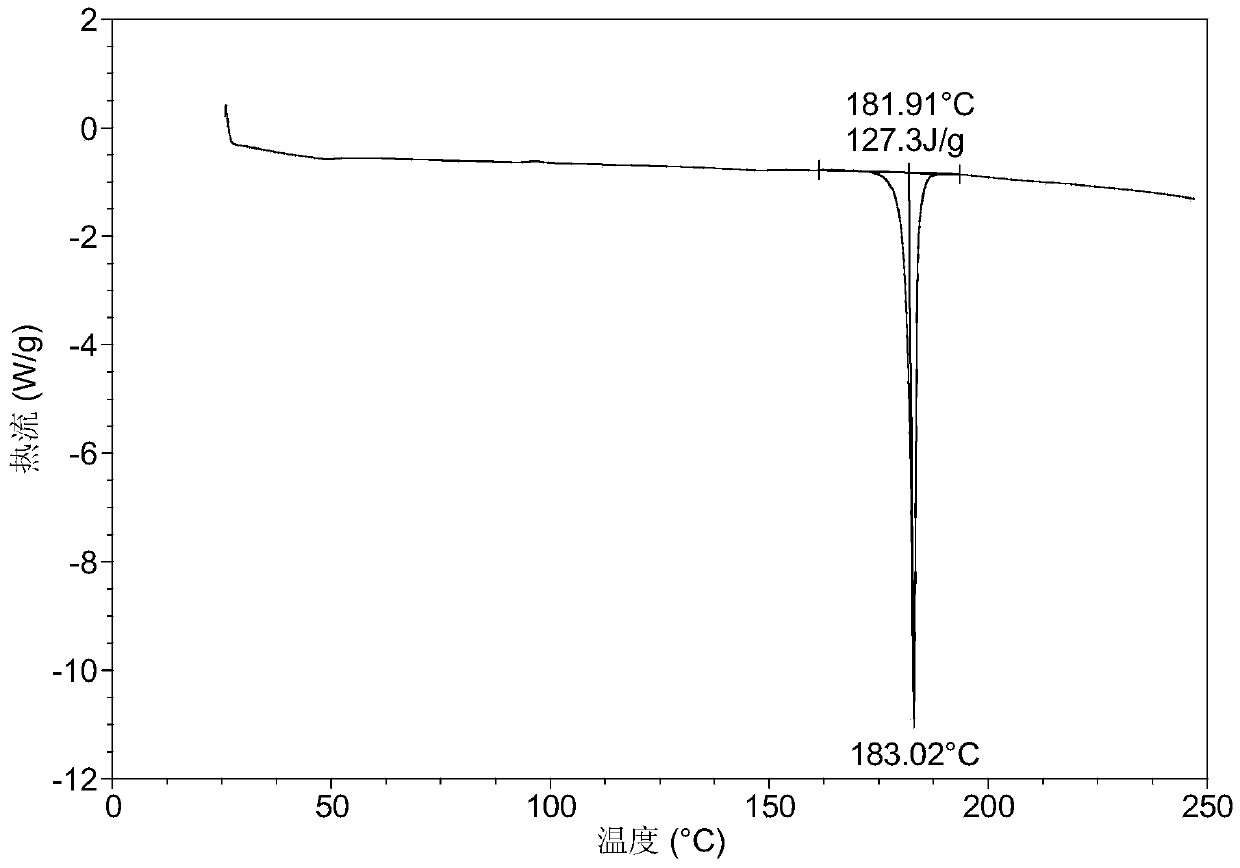
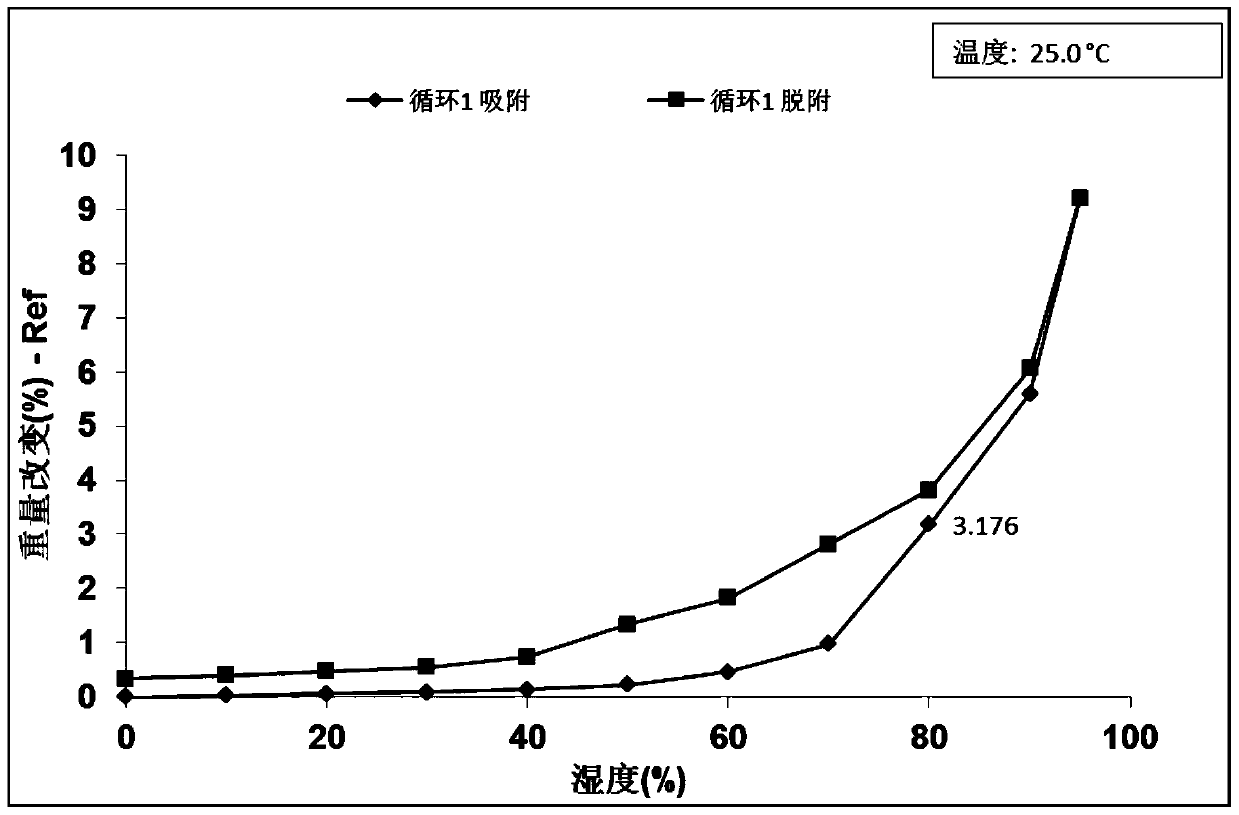
A B crystal form of 2-[(2R)-2-methyl-2-pyrrolidinyl]-1H-benzimidazole-7-carboxamide (ABT-888), a preparation method thereof and use are provided. The X-ray powder diffraction pattern (CuKα radiation) of B crystal form at 25 ℃ has characteristic peaks at 2 theta values of 9.40 ° ± 0.20 °, 17.30 ° ± 0.20 °, 22.80 ° ± 0.2 °. The B crystal form is prepared by dissolving ABT-888 free alkali in methanol and volatilizing the same at room temperature into a B crystal form. Compared with existing crystal forms, the B crystal form has a higher solubility and a simpler preparation process, and has good stability. The B crystal form is of great significance for improving the efficacy of and for reducing the drug load for treating metastatic breast cancer, colon cancer, metastatic melanoma and brain tumor.
Description
Technical field [0001] The invention relates to the crystal form B of 2-[(2R)-2-methyl-2-pyrrolidinyl]-1H-benzimidazole-7-carboxamide and a preparation method thereof. Background technique [0002] 2-[(2R)-2-Methyl-2-pyrrolidinyl]-1H-benzimidazole-7-carboxamide (compound of formula I), also known as ABT-888 (veliparib), is produced by Abbott ( Abbvie) a new type of high-selectivity PARP inhibitor developed by the company. In vivo and in vitro experiments have shown that ABT-888 has a significant inhibitory effect on PARP activity, and has achieved significant effects in the treatment of metastatic breast cancer, colon cancer, metastatic melanoma and brain tumors. At present, the combined application of ABT-888 and whole brain radiotherapy for the treatment of metastatic brain tumors has entered the phase I clinical stage, and the treatment of metastatic breast cancer, colon cancer and metastatic melanoma has also entered the phase II clinical stage, and is used in combination wi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D403/04A61K31/4184A61P35/00
CPCC07B2200/13C07D403/04A61K31/4184
Inventor 陈敏华张炎锋刘凯张晓宇
Owner CRYSTAL PHARMATECH CO LTD
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com