Application of Terreumol A in preparation of neuroprotective drug
A technology of terreumola, 1. terreumola is applied in the application field of terreumolA in the preparation of neuroprotective drugs, and can solve problems such as no compound activity report yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Isolation, Preparation and Structure Confirmation of Terreumol A
[0013] The preparation method of Terreumol A is the same as that reported in the literature (HighlyOxygenatedMeroterpenoidsfromFruitingBodiesoftheMushroomTricholomaterreum, J.Nat.Prod., 2013, 76, 1365-1368).
[0014] Structural confirmation: yellow crystal, molecular formula is C 25 h 34 o 8 , with an unsaturation of 8. The IR data show that the compound contains a hydroxyl group (3440cm - 1 ), the compound has ultraviolet absorption at 372 and 291nm, indicating that it contains a conjugated moiety. H NMR spectrum data δ H (ppm, DMSO-d 6 , 600MHz): H-2 (6.52, s), H-5 (3.44, d, J=15.1), H-5 (2.77, d, J=15.1), H-7 (2.85, dd, J=9.8 , 3.8), H-8 (2.27, m), H-8 (1.45, m), H-9 (2.33, dd, J=12.7, 7.3), H-9 (1.43, m), H-11 ( 3.89, m), H-15 (1.33, s), H-16 (1.38, s), 1-OH (11.97, s), 4-OH (7.85, s), 3-OCH 3 (3.96, s); C NMR spectrum data δ C (ppm, DMSO-d 6 , 600Hz): 159.2 (C, 1-C), 99.7 (CH,...
Embodiment 2
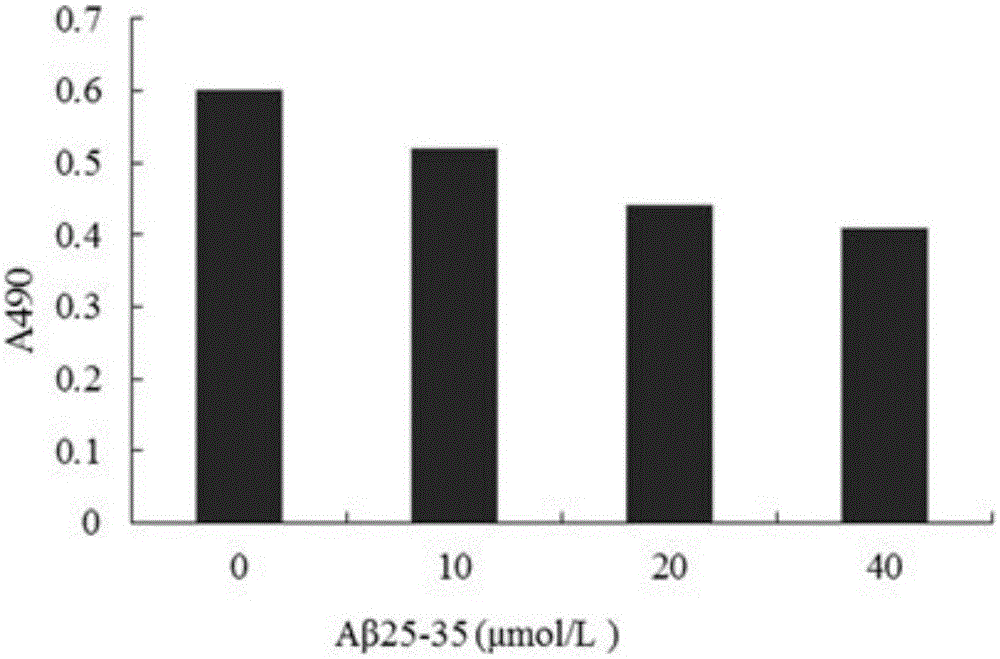
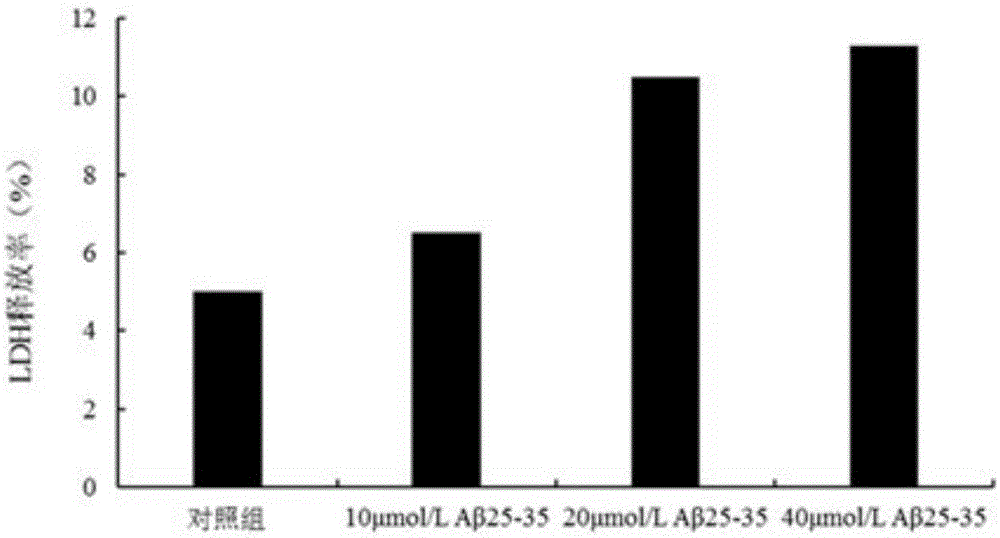
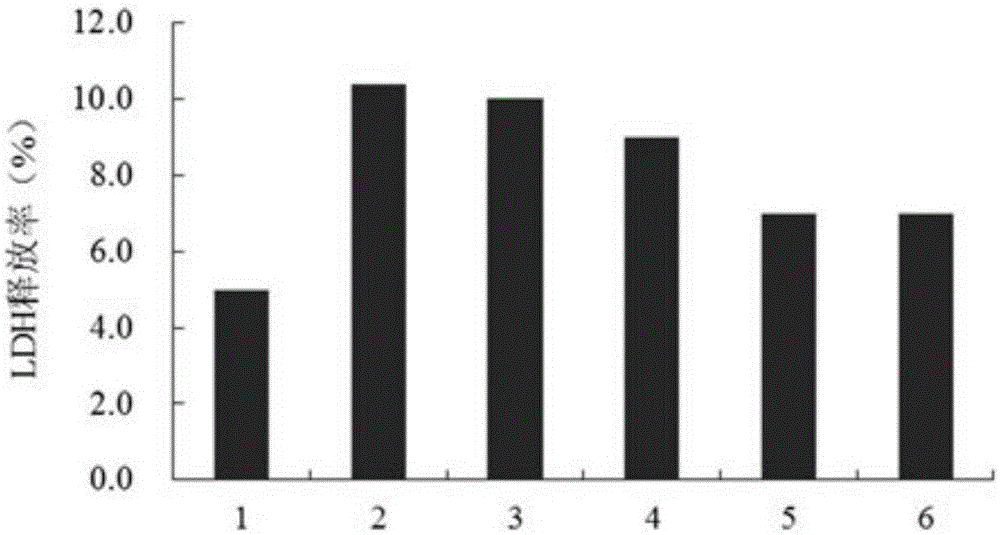
[0015] Embodiment 2: The pharmacological action test of TerreumolA
[0016] 1. Materials and Instruments
[0017] Aβ 25-35 Purchased from sigma company in the United States. TerreumolA is self-made, and the HPLC normalized purity is greater than 98%. MTT (nitroblue tetrazolium) was purchased from Amresco, USA. LDH assay kit was purchased from Nanjing Jiancheng Biological Co., Ltd. Acridine orange (AO) was purchased from Sigma, USA. Ethidium bromide (EB) was purchased from Sigma, USA. RNase enzyme was purchased from Sigma, USA. Proteinase K was purchased from sigmaAnnexin, USA. Ⅴ-FITC cell apoptosis detection kit was purchased from Nanjing Kaiji Biotechnology Development Co., Ltd. The dry powder of D-MEM / F12 medium was purchased from GibcoL, USA. Horse serum was purchased from hycLon Company in the United States. Fetal bovine serum was purchased from Hangzhou Sijiqing Biological Engineering Co., Ltd. Polylysine (PLL) was purchased from Sigma, USA.
[0018] Low-tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com