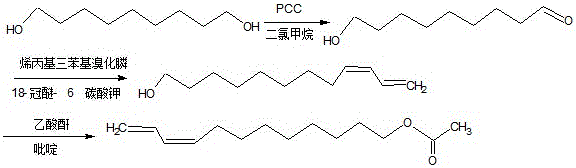
The synthetic method of z9,11-dodecadienol acetate
A technology of carbadienol acetate and dodecadienol, which is applied in the preparation of carboxylic acid esters, carbon-based compounds, chemical instruments and methods, etc., and can solve the problem of harsh reaction conditions, accidents, and synthetic routes long-term problems, to achieve the effect of mild synthesis conditions, low cost, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Synthesis of 9-hydroxyl-1-nonanal
[0016] In a 500mL standard ground three-neck flask equipped with a reflux condenser, add 5.4g of 1,9-nonanediol and 81mL of anhydrous dichloromethane, stir electrically, and under the protection of nitrogen, add 7.02 g PCC and 7.02g silica gel, after adding all the reactants, continue to react at room temperature for 1h, then add 16.2mL distilled water and then add 81mL ether, stir and extract for 40min, then use silica gel to suction filter, transfer the filtrate to a separatory funnel, add dropwise 20% sodium hydroxide made the filtrate neutral, extracted three times with ether and combined the organic phases, washed three times with saturated sodium chloride, dried over anhydrous sodium sulfate for 12 hours, evaporated the solvent, and separated the residue with a silica gel column to obtain 9-hydroxyl-1 - Nonanal 4.0g, the productive rate is 74.1%.
[0017] (2) Synthesis of Z9,11-dodecadienol
[0018] In a 250mL standard gro...
Embodiment 2
[0022] (1) Synthesis of 9-hydroxyl-1-nonanal
[0023] In a 500mL standard ground three-neck flask equipped with a reflux condenser, add 8.0g of 1,9-nonanediol and 96mL of anhydrous dichloromethane, stir electrically, and under nitrogen protection, add 12.8 g PCC and 12.8g silica gel, after adding all the reactants continue to react at room temperature for 3h, then add 28.8mL distilled water and then add 96mL diethyl ether, stir and extract for 60min, then use silica gel to suction filter, transfer the filtrate to a separatory funnel, add dropwise 20% sodium hydroxide made the filtrate neutral, extracted three times with ether and combined the organic phases, washed three times with saturated sodium chloride, dried over anhydrous sodium sulfate for 12 hours, evaporated the solvent, and separated the residue with a silica gel column to obtain 9-hydroxyl-1 - Nonanal 5.51g, the productive rate is 68.9%.
[0024] (2) Synthesis of Z9,11-dodecadienol
[0025] In a 250mL standard gr...
Embodiment 3
[0029] (1) Synthesis of 9-hydroxyl-1-nonanal
[0030] Add 7.5g of 1,9-nonanediol and 105mL of anhydrous dichloromethane into a 500mL standard three-neck flask with a reflux condenser, add 10.5 mL of anhydrous dichloromethane in three batches under the protection of nitrogen g PCC and 10.5g silica gel, after all the reactants were added, the reactant continued to react at room temperature for 2h, then added 25mL distilled water and then 105mL diethyl ether, stirred and extracted for 50min, then filtered with silica gel, transferred the filtrate to a separatory funnel, added dropwise 20 % sodium hydroxide to make the filtrate neutral, extracted three times with ether and combined the organic phases, washed three times with saturated sodium chloride, dried over anhydrous sodium sulfate for 12 hours, evaporated the solvent, evaporated the solvent, and the residue was separated with a silica gel column to obtain 9- Hydroxy-1-nonanal 5.44g, the yield is 72.6%.
[0031] (2) Synthesis ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com