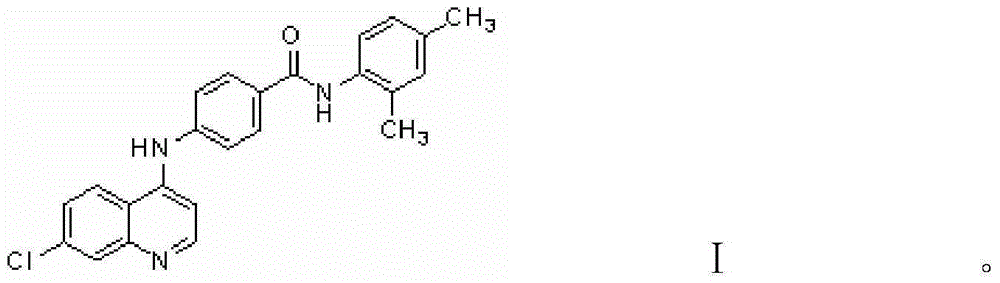
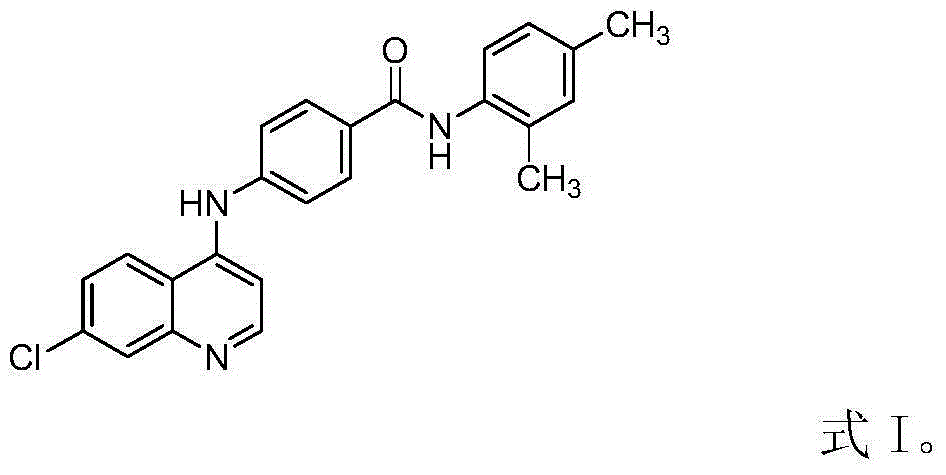
Substance with protein kinase inhibiting activity and preparation method therefor and application thereof
A protein kinase inhibition and activity technology, applied in the field of medicine, can solve the problems of harsh reaction conditions, high prices, and unsuitable compounds for industrial production, and achieve the effects of simple and easy control of the reaction process, low cost of the preparation method, and obvious anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the benzamide derivatives involved in the present invention is as follows:
[0029] The following room temperature refers to 25°C, and ice bath refers to 0-4°C.
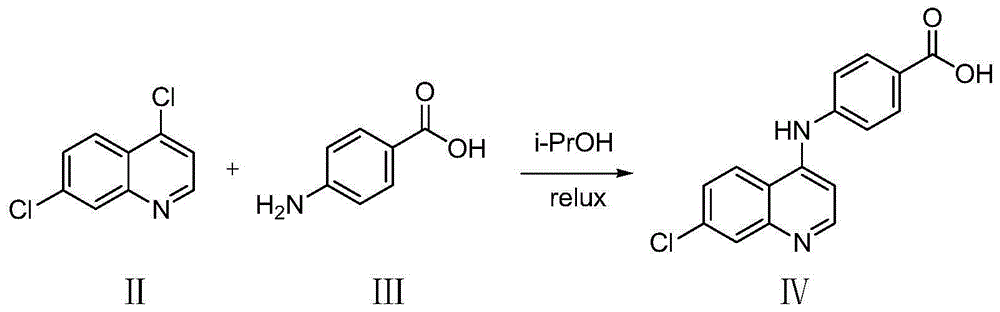
[0030] Step (1), Dissolving Compound II (4,7-dichloroquinoline) (5-20mmol) and Compound III (p-aminobenzoic acid) (5-20mmol) in isopropanol (20-50mL), stirred and refluxed (75-95°C), after 5-7 hours, a pale yellow precipitate precipitated, after the reaction solution was cooled to room temperature, evaporated to dryness under reduced pressure, the residual solid was washed 3 times with dichloromethane, the filter cake was collected, and dried to obtain a pale yellow solid Powder IV (4-(7-chloroquinoline-4-amino)benzoic acid).
[0031] The reaction equation is as follows:
[0032]
[0033] Step (2), dissolve the compound IV obtained in step (1) in dry dichloromethane, stir, slowly add thionyl chloride (25-100mmol) dropwise to the reaction system under ice bath, and reflux after th...
Embodiment 1
[0042] Add 4,7-dichloroquinoline (0.985g, 5mmol), p-aminobenzoic acid (0.685g, 5mmol) and 20mL of isopropanol into a dry 100mL round-bottomed flask, and stir to fully dissolve it. Slowly raise the temperature to 85°C, stir and reflux for 5 hours, the reaction solution precipitates a light yellow precipitate, TLC detects that the reaction has been balanced, and the reaction is terminated. After the reaction solution was cooled to room temperature, it was evaporated to dryness under reduced pressure, the residual solid was washed 3 times with 10 mL of dichloromethane, the filter cake was collected, and dried to obtain 1.40 g of light yellow solid powder 4-(7-chloroquinoline-4-amino)benzoic acid , yield 95%.
[0043] Add 10 mL of dichloromethane solution containing 4-(7-chloroquinoline-4-amino)benzoic acid (1.491 g, 5 mmol) into a 100 mL dry round bottom flask, stir, and slowly add Add thionyl chloride (2.974g, 25mmol) dropwise, reflux (80°C) for 4 hours after the dropwise addit...
Embodiment 2
[0051] Add 4,7-dichloroquinoline (1.970g, 10mmol), p-aminobenzoic acid (1.372g, 10mmol) and 20mL of isopropanol into a dry 100mL round-bottomed flask, and stir to fully dissolve it. Slowly raise the temperature to 85°C, stir and reflux for 5 hours, the reaction solution precipitates a light yellow precipitate, TLC detects that the reaction has been balanced, and the reaction is terminated. After the reaction solution was cooled to room temperature, it was evaporated to dryness under reduced pressure, the residual solid was washed 3 times with 15 mL of dichloromethane, the filter cake was collected, and dried to obtain 2.751 g of light yellow solid powder 4-(7-chloroquinoline-4-amino)benzoic acid , yield 92%.
[0052] Add 15 mL of dry methylene chloride solution containing 4-(7-chloroquinoline-4-amino)benzoic acid (2.982 g, 10 mmol) into a 100 mL dry round bottom flask, stir, and pour into the reaction system under an ice bath at 0°C Slowly add thionyl chloride (5.948g, 50mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com