Application of sulfonamide compound in chronic airway inflammatory diseases
A technology of chronic inflammation and general formula compounds, which is applied in the medical field and can solve the problems of not being able to apply to all types of diseases and unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
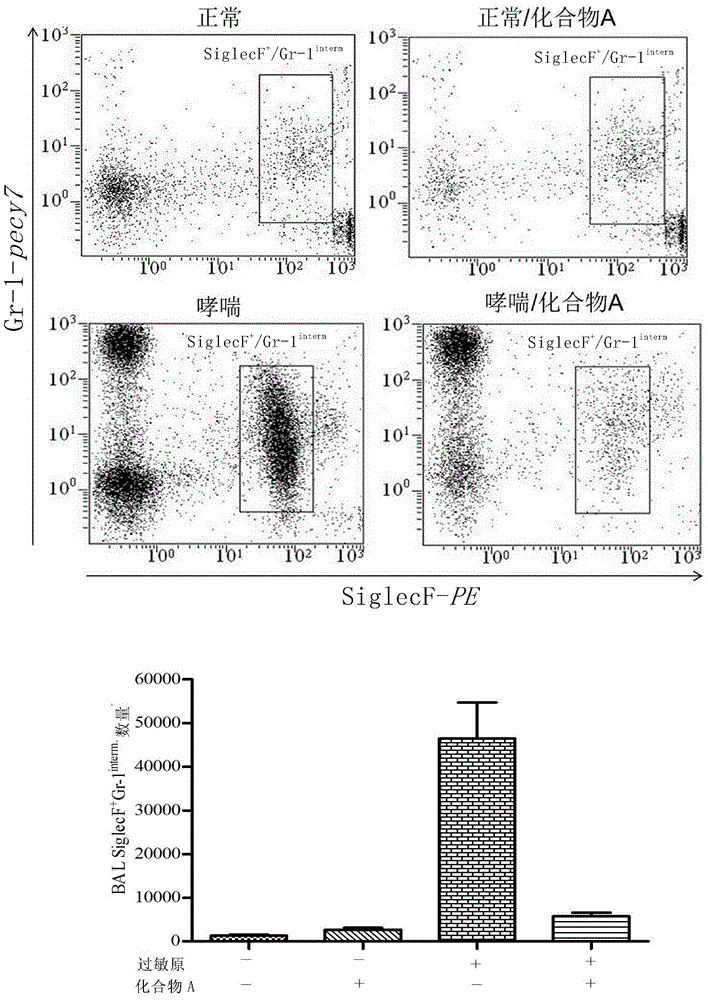
[0139] Example 1 Effect of Compound A on eosinophil infiltration-based airway inflammation:
[0140] method:
[0141] 1) Choose 8-week-old healthy mice;
[0142] 2) Sensitization: mice were intraperitoneally injected with sensitization solution on days 0 and 14;
[0143] 3) Allergen atomization challenge: on the 24th, 25th, and 26th day, put the mice in a closed container, and inhale the allergen solution for 40 minutes, for three consecutive days.
[0144] 4) The control group was sensitized and aerosolized with the same dose of normal saline instead of allergens.
[0145] 5) Compound A airway topical drug treatment:
[0146] Ⅰ) Administration time: 4 hours after the allergen aerosol challenge on the 24th, 25th, and 26th day, for three consecutive days;
[0147] Ⅱ) Dosage: Compound A100ug / mouse;
[0148] Ⅲ) Administration method: airway administration in mice under anesthesia;
[0149] Ⅳ) The control group was administered with the same dose of Compound A solvent airway...
Embodiment 2
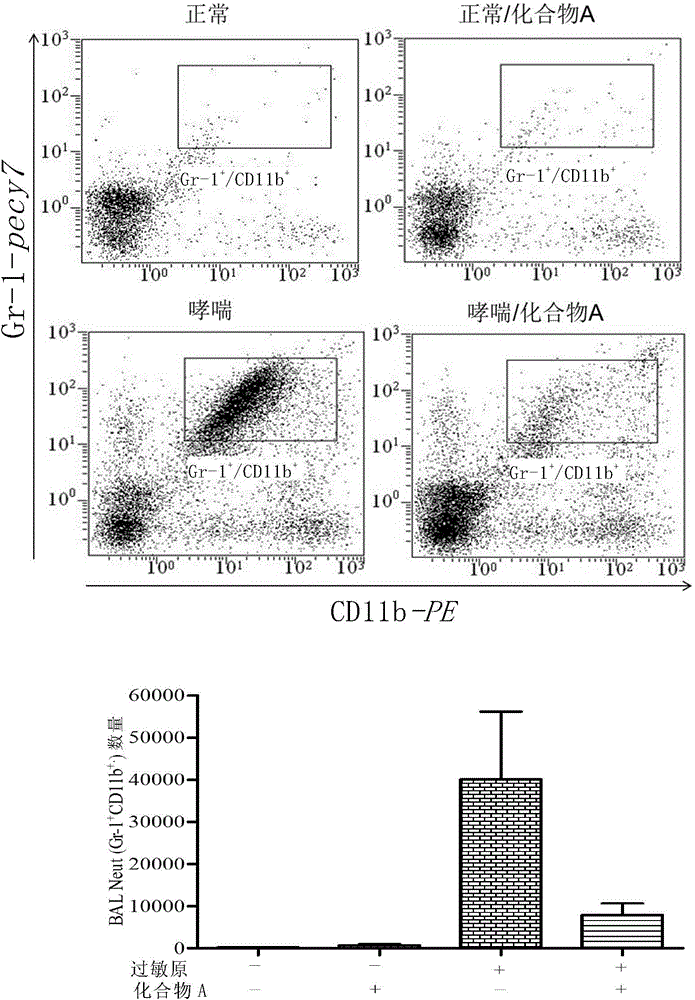
[0154] Example 2 Effect of Compound A on neutrophil infiltration-based airway inflammation:
[0155] method:
[0156] 1) Choose 8-week-old healthy mice;
[0157] 2) Sensitization: mice were intraperitoneally injected with sensitization solution on day 0;
[0158] 3) Allergen atomization challenge: on the 14th day, put the mice in a closed container, and inhale the allergen solution by atomization for 40 minutes.
[0159] 4) The control group was sensitized and aerosolized with the same dose of normal saline instead of allergens.
[0160] 5) Compound A airway topical drug treatment:
[0161] Ⅰ) Administration time: 4 hours after allergen aerosol challenge on the 14th day;
[0162] Ⅱ) Dosage: Compound A100ug / mouse;
[0163] Ⅲ) Administration method: airway administration in mice under anesthesia;
[0164] Ⅳ) The control group was administered with the same dose of Compound A solvent airway.
[0165] result:
[0166] (1) Airway administration of Compound A inhibited the r...
Embodiment 3
[0168] The dose selection of embodiment 3Compound A
[0169] 1) Choose 8-week-old healthy mice;
[0170] 2) Sensitization: mice were intraperitoneally injected with sensitization solution on day 0;
[0171] 3) Allergen atomization challenge: on the 14th day, put the mice in a closed container, and inhale the allergen solution by atomization for 40 minutes.
[0172] 4) The control group was sensitized and aerosolized with the same dose of normal saline instead of allergens.
[0173] 5) Compound A airway topical drug treatment:
[0174] Ⅰ) Administration time: 4 hours after allergen aerosol challenge on the 14th day;
[0175] Ⅱ) Dosage: Group A: Compound A50ug / mouse, Group B: Compound A100ug / mouse; Group C: Compound A250ug / mouse; Group D: Compound A500ug / mouse; Group E: Compound A1000ug / mouse.
[0176] Ⅲ) Administration method: airway administration in mice under anesthesia;
[0177] Ⅳ) The control group was administered with the same dose of Compound A solvent airway.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com