Primer and method for simultaneously detecting polymorphism of CYP2C*2 and CYP2C*3 genes
A gene polymorphism and genotyping technology, applied in biochemical equipment and methods, microbial measurement/inspection, DNA/RNA fragments, etc., can solve the problems of high cost of gene chip design, high detection cost, lack of specificity, etc. , to achieve good accuracy, high sensitivity, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Primers
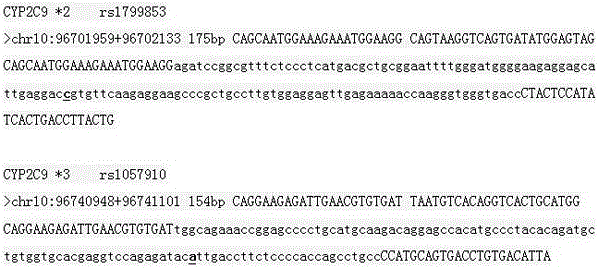
[0021] The inventor designed a large number of primers for the gene polymorphism sites of CYP2C9*2 and CYP2C9*3. Through optimization and comparison of the primer reaction conditions, the primers were screened out with good specificity, no cross-reaction and very close PCR reaction conditions. primers. The primers provided by the present invention are shown in Table 1, including PCR amplification primers and SNaPshot PCR primers, and the PCR amplification primers and SNaPshot PCR primers are corresponding. All primer sequences provided by the present invention are compared through UCSC database, and there is no known SNP site.
[0022]
Embodiment 2
[0023] The specificity of embodiment two primers
[0024] The primers provided by the present invention were blasted in UCSC, and the results were as follows: CYP2C9 *2 The amplified fragment is located between chr10:96701959-96702133, with a length of 175bp; CYP2C9 *3 The amplified fragment is located at chr10:96740948-96741101, with a length of 154bp; the result is as follows figure 1 As shown, the amplified fragments of all primers covered the corresponding detection sites: CYP2C9 *2 and CYP2C9 *3, No other homologous genes.
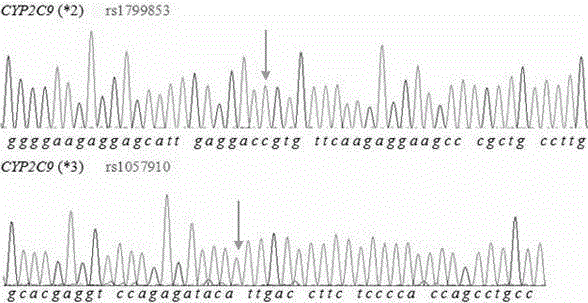
[0025] The PCR amplification primers in Table 1 were used to amplify and Sanger sequence the detection samples respectively. The sequencing results showed that the amplified fragments of each primer were the same as CYP2C9 *2 and CYP2C9 *3 The gene reference sequence matches, the result is as follows figure 2 shown. Using the SNaPshot PCR primers in Table 1, the SNaPshot method is used for detection, and the results are as follows im...
Embodiment 3
[0033] Example three detection CYP2C9 *2 and CYP2C9 *3 Specificity of the method for genetic polymorphism
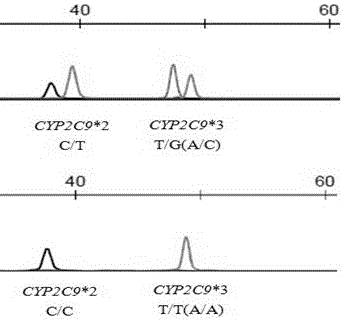
[0034] The specificity of this assay is defined as the negative coincidence rate. The CYP2C9*2 430 C / C genotype accounted for the vast majority, and the CYP2C9*3 1075A / A genotype accounted for the vast majority. Therefore, in this test, the detection of CYP2C9*2 430 C / C genotype is defined as a negative result, and the detection of CYP2C9*3 1075 A / A genotype is defined as a negative result.
[0035] The SNaPshot method provided by the present invention was used to detect 18 samples, and at the same time, the Sanger sequencing method was used for verification. The SNaPshot sequencing method detected a total of 11 samples without mutation (negative), which was consistent with the results shown by the Sanger sequencing method, as shown in Table 4. The specificity of this detection method is 100%.
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com