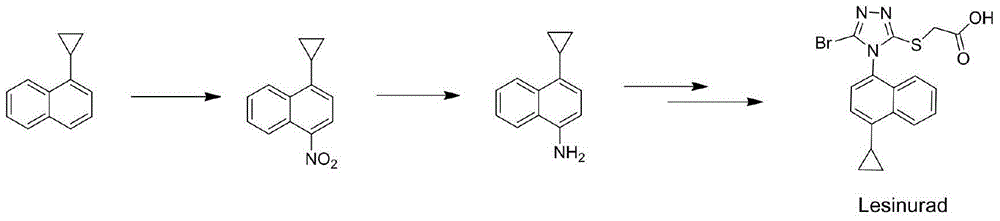
Preparation process for gout suppressant Lesinaurad intermediates
A preparation process and neutral technology are applied in the field of preparation technology of an intermediate of anti-gout drug Lesinaurad, which can solve the problems of difficult industrial production and high raw material prices, and achieve the effects of low cost, high total yield, and cheap and easy-to-obtain raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0034] Experimental example 1: the screening of alkali in step (1):
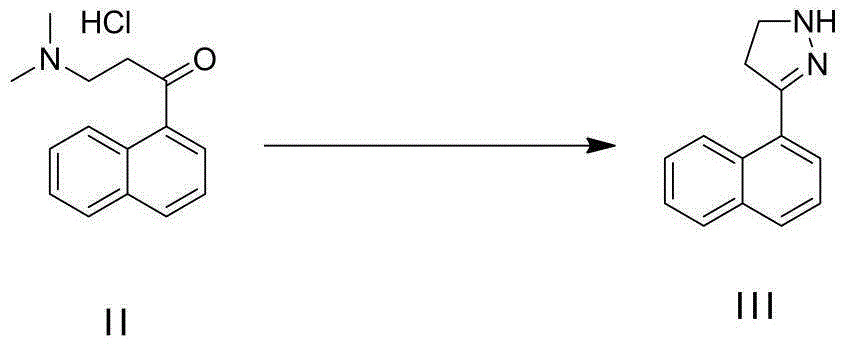
[0035] Add 240G of ethanol into a 500mL three-necked flask, then start stirring, then add 100G of 3-(dimethylamino)-1-(naphthalene-1-yl)-1-acetone hydrochloride, after the addition is complete, heat up to 45- At 50°C, 0.05KG of hydrazine hydrate and the corresponding base (3.5 equivalents) were added dropwise. After the feeding is completed, the temperature is raised to 65-70°C. After the reaction reaches the end point, the temperature of the reaction solution is cooled to 30-40°C, the liquid is separated, the upper organic phase is collected, concentrated to dryness, and compound III is obtained. The reaction data is as follows:
[0036]
[0037] As can be seen from the data in the table above: the reaction effect of NaOH and KOH is better, but the reaction yield is slightly higher with NaOH as alkali, and the price of NaOH is cheaper than KOH, therefore, alkali is preferably NaOH in the step (1).
experiment example 2
[0038] Experimental example 2: the screening of catalyst in the step (2):
[0039] Add 240G of ethanol into a 500mL three-necked flask, then start stirring, then add 100G of 3-(dimethylamino)-1-(naphthalene-1-yl)-1-acetone hydrochloride, after the addition is complete, heat up to 45- At 50°C, 0.05KG of hydrazine hydrate and the corresponding base (3.5 equivalents) were added dropwise. After the feeding is completed, the temperature is raised to 65-70°C. After the reaction reaches the end point, the temperature of the reaction solution is cooled to 30-40°C, the liquid is separated, the upper organic phase is collected, concentrated to dryness, and compound III is obtained.
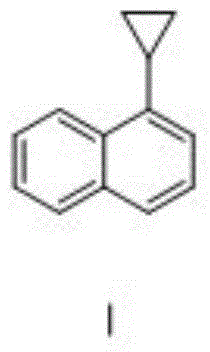
[0040] Put the above oil into a 2L single-necked bottle, add the corresponding catalyst, and heat to 150-210°C for cracking reaction. After the reaction is complete for a certain period of time, distill under reduced pressure to obtain cyclopropylnaphthalene. The experimental data are as follows:
[0041]...
Embodiment 1
[0044] Add 15KG of ethanol into the 50L reaction kettle, then start stirring, then add 10KG of 3-(dimethylamino)-1-(naphthalene-1-yl)-1-acetone hydrochloride, after the addition is complete, heat up to 45- At 50°C, add dropwise a mixture of 3.8KG hydrazine hydrate and (1.75KG sodium hydroxide / 1.75KG water / 6.5KG methanol). After the feeding is completed, the temperature is raised to 65-70°C. After the reaction reaches the end point, the ethanol is concentrated under reduced pressure, 13KG of MTBE is added, stirred and cooled to 20-25°C, filtered, the filter cake is rinsed with MTBE (5KG), and the filtrates are combined. Separate the layers, wash the organic phase with 2KG saturated brine, distill the organic phase under normal pressure until no liquid flows out, stop the concentration, and put the obtained oil directly into the next reaction.
[0045] Add the above-mentioned oil to a 20L high-temperature kettle, and heat it to 150-190°C for cracking reaction. After 5 hours, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com