Multivalent meningococcal preparation kit, vaccine preparation and preparation method thereof
A meningococcal and vaccine technology, applied in antibacterial drugs, pharmaceutical formulations, bacterial antigen components, etc., can solve the problems of complicated vaccine production process, difficult multi-component antigen content analysis and quality control, and achieve freeze-drying process optimization , maintain high activity, optimize the effect of solvent parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of meningococcal polysaccharides or polysaccharide-protein-bound stock solutions of groups A, C, W135, and Y
[0039] After recovery, the working seeds of group A, C, W135, and Y meningococci were inoculated with the fermentation medium, and the bacterial cells were collected by centrifugation after fermentation. Discard the cell pellet, and separate and purify the capsular polysaccharide antigen from the supernatant.
[0040] The purification of polysaccharides adopts steps such as ultrafiltration concentration, membrane bag washing, and gel separation to remove phenol, chloroform, protease and other reagents that are obviously harmful to the human body used in traditional processes.
[0041] The purified polysaccharides that pass the test are diluted according to a certain ratio and then prepared to obtain the stock solution of meningococcal polysaccharides of groups A, C, W135, and Y.
[0042] Before the polysaccharide-protein binding stock solution is p...
Embodiment 2
[0046] Preparation of Quadrivalent Meningococcal Vaccine (Polysaccharide Protein Conjugate Vaccine) Preparation
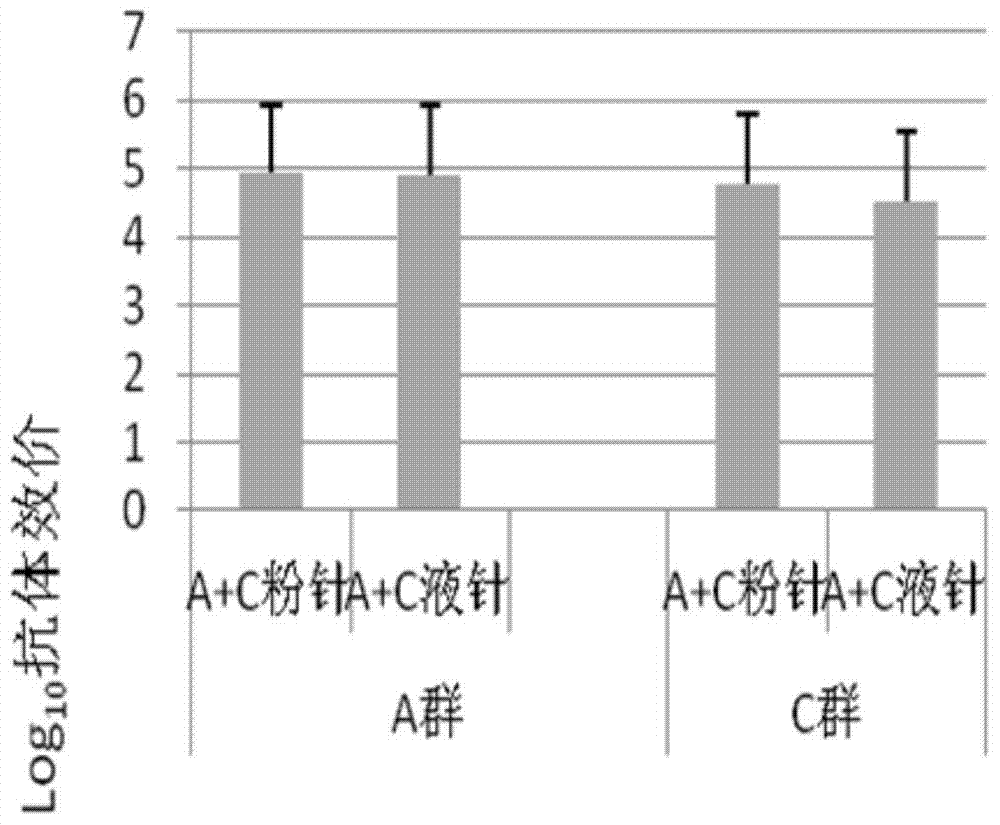
[0047] Take 10 μg of group A meningococcal polysaccharide-CRM197 conjugate stock solution and 10 μg of group C meningococcal polysaccharide CRM197 conjugate stock solution in a vial, add lyoprotectant (20 mg mannitol and 5 mg sucrose), and half stopper Then put it into the cavity of the freeze dryer, pre-freeze at -45°C for 240 to 300 minutes, reduce the temperature to -35°C to vacuum, keep the vacuum at 0.2±0.02mBar, and vacuum at -35°C Dry for 840 to 960 minutes, heat up to 35°C, and dry for 300 to 600 minutes to prepare a freeze-dried component;
[0048] Take 10 μg of Y group meningococcal polysaccharide-CRM197 conjugate stock solution and 10 μg W135 meningococcal polysaccharide CRM197 conjugate stock solution in a vial, add 10 mmol phosphate buffer and 0.45% physiological sodium chloride solution A diluent composed of a mixture of diluents, prepared as a liqui...
Embodiment 3
[0053] Preparation of Quadrivalent Meningococcal Vaccine (Polysaccharide Vaccine) Preparation
[0054] Take 50 μg of group A meningococcal polysaccharide stock solution and 50 μg of group C meningococcal polysaccharide stock solution into a vial, add a freeze-drying protective agent (20 mg of mannitol and 5 mg of sucrose), put it into the cavity of a freeze dryer after half stoppering, Pre-freeze at -45°C for 240 to 300 minutes, lower the temperature to -35°C to vacuum, maintain a vacuum of 0.2±0.02mBar, vacuum dry at -35°C for 840 to 960 minutes, and heat up to 35°C. Ordinary drying for 300-600 minutes to prepare freeze-dried components;
[0055] Take 50 μg Y group meningococcal polysaccharide stock solution and 50 μg W135 group meningococcal polysaccharide stock solution and place them in a vial, add a diluent consisting of a mixture of 10 mmol phosphate buffer saline and 0.45% physiological sodium chloride solution, and prepare a dilution containing the liquid component of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com