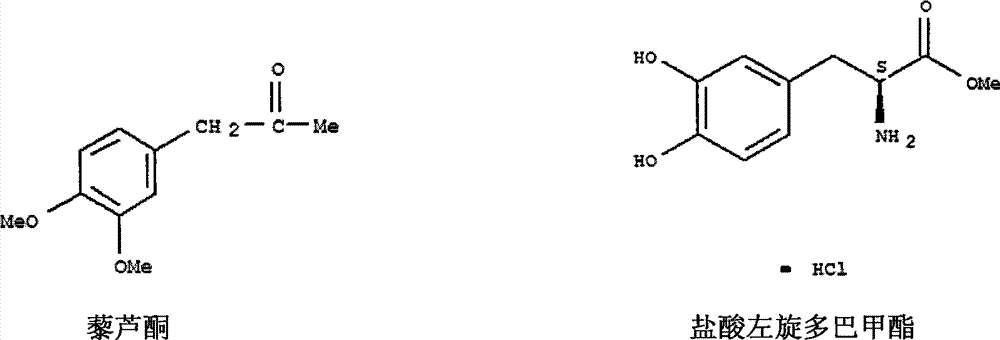
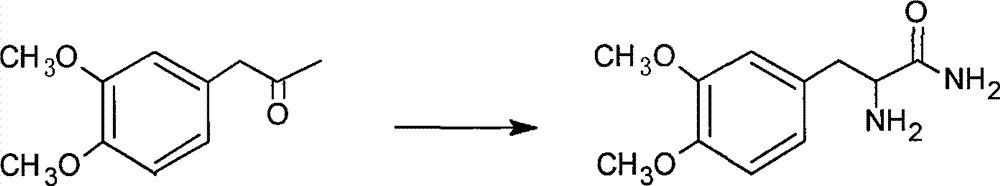
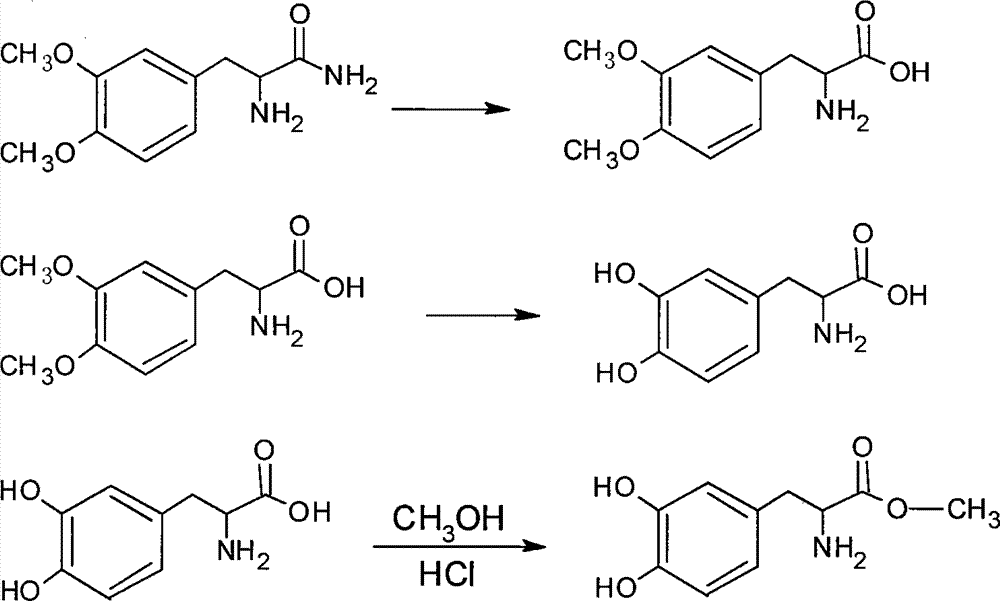
A kind of synthetic method of levodopa methyl ester hydrochloride
A synthesis method and dopa methyl ester technology are applied in the synthesis field of levodopa methyl ester hydrochloride, and can solve the problems such as toxicity of LDME
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The synthetic route of the present invention is as follows:
[0016]
[0017]
[0018] 1. The preparation of 2-amino-3-(3,4-dimethoxyphenyl)propanamide was equipped with mechanical stirring, thermometer, 500mL four-neck round bottom flask with drying tube and reflux condenser, adding 20g Veratrone and 30g sodium hydroxide, 10g potassium chloride and 90mL concentrated ammonia water, add 23g triethylbenzyl ammonium chloride under stirring, cool to 0-5°C, feed ammonia gas, and keep the reaction at 0-5°C Left and right, place at room temperature for 24 hours, then stir for 3 hours, add 200mL of water and stir thoroughly, extract the aqueous layer with ethyl acetate for 3-5 times, combine the ethyl acetate extracts, recover the ethyl acetate solvent under reduced pressure, and extract the concentrate under reduced pressure Dry to obtain viscous matter, add a small amount of dichloromethane, heat and stir to dissolve, filter after cooling to obtain crude 2-amino-3-(3,4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com