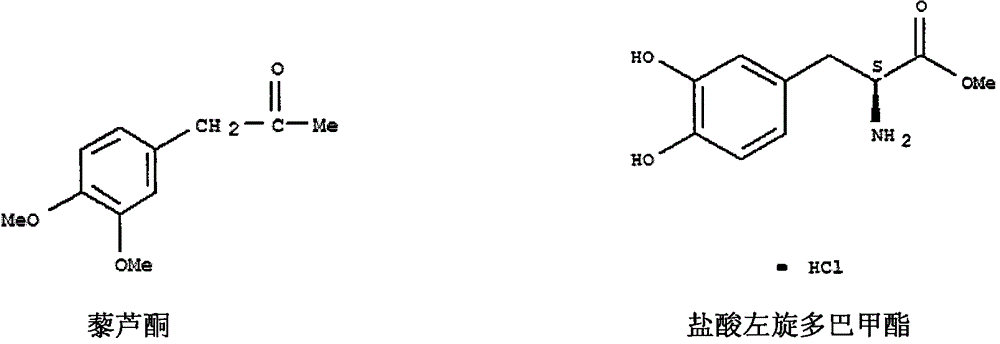
Synthesis method of L-dopa methyl ester hydrochloride
A technology of levodopa methyl ester and synthesis method, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problem of LDME toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
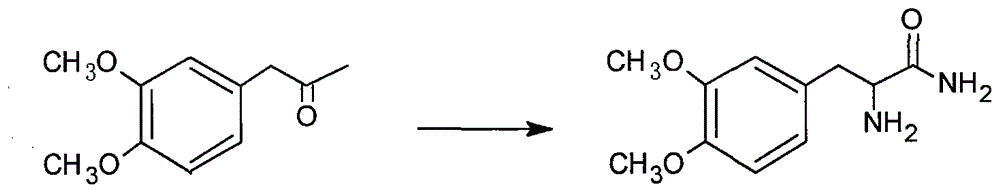
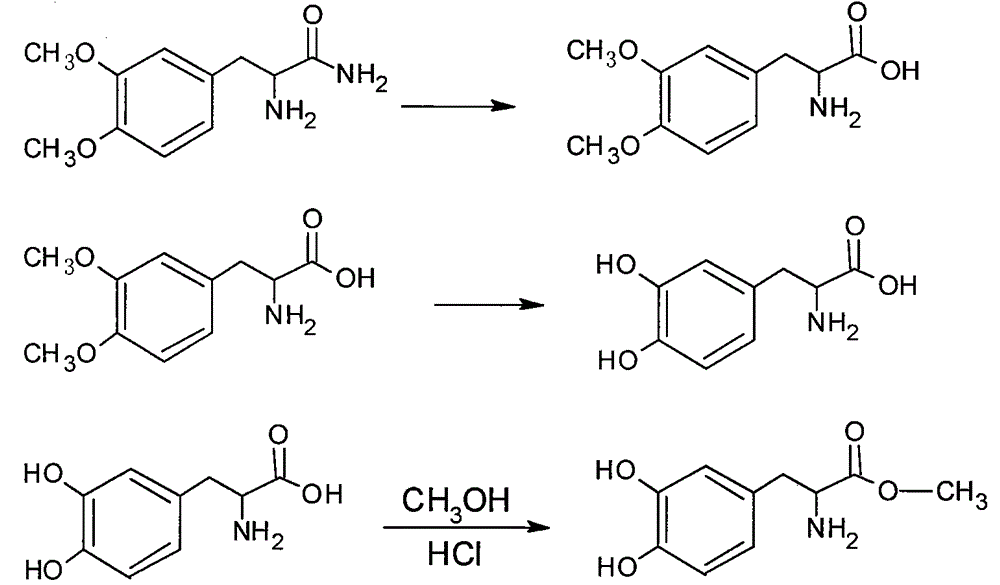
[0015] The synthetic route of the present invention is as follows:
[0016]
[0017]
[0018] 1. The preparation of 2-amino-3-(3,4-dimethoxyphenyl)propanamide was equipped with mechanical stirring, thermometer, 500mL four-neck round bottom flask with drying tube and reflux condenser, adding 20g Veratrone and 30g sodium hydroxide, 10g potassium chloride and 90mL concentrated ammonia water, add 23g triethylbenzyl ammonium chloride under stirring, cool to 0-5°C, feed ammonia gas, and keep the reaction at 0-5°C Left and right, place at room temperature for 24 hours, then stir for 3 hours, add 200mL of water and stir thoroughly, extract the aqueous layer with ethyl acetate for 3-5 times, combine the ethyl acetate extracts, recover the ethyl acetate solvent under reduced pressure, and extract the concentrate under reduced pressure Dry to obtain viscous matter, add a small amount of dichloromethane, heat and stir to dissolve, filter after cooling to obtain crude 2-amino-3-(3,4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com