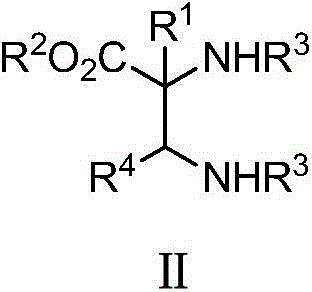
Alpha, beta-diamino acid derivative with optically active alpha-quaternary carbon and preparation method and application thereof
An optically active, diamine acid technology, applied in the α field of α-position quaternary carbon, can solve problems such as low efficiency, poor economy, limited substrate scope, etc., and achieve the effects of simple synthetic route, high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
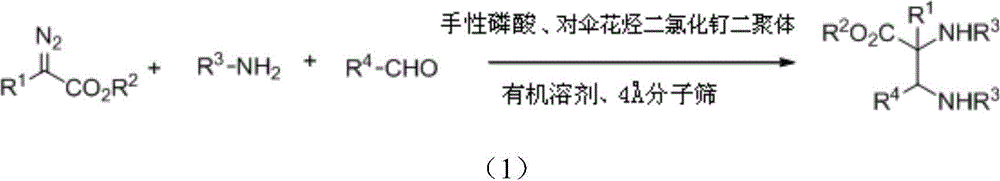
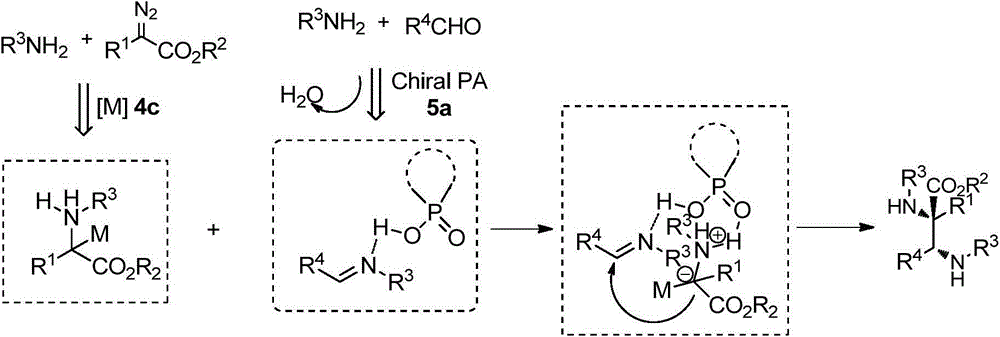
[0039] The preparation method of the present invention is as follows:
[0040] First weigh the amine: aldehyde: chiral phosphoric acid: p-cymene dichloride ruthenium dimer = 1.0: 1.0: 0.05: 0.01 in molar ratio, and add to the pre-dried round bottom flask in sequence Amines, aldehydes, chiral phosphoric acid catalysts, p-cymene dichloride ruthenium dimers and water absorbents Molecular sieve, water absorbent The amount of molecular sieve added is 1-2g / mmol aldehyde, and organic solvent is added to the round bottom flask at room temperature, the amount of organic solvent is 20-30ml / mmol aldehyde; the round bottom flask is stirred at room temperature for 1h, and then put into Stir in a low-temperature reaction bath at 10°C for 1 hour. Then, weigh the diazo:amine=1.1:1.0 by molar ratio, put the diazo and amine into a small bottle, and dissolve it with an organic solvent to dissolve diazoamine The amount of the organic solvent is 20-30ml / mmol aldehyde, stir evenly to obtain the...
Embodiment 1
[0042]
[0043] Weigh 2,5-dimethoxyaniline (15.3mg, 0.1mmol), p-cymene dichloride ruthenium dimer (1.2mg, 0.00lmmol), (S)-3,3'-triphenyl Silicon-substituted BINOL phosphoric acid (4.3mg, 0.005mmmol), p-bromobenzaldehyde (18.5mg, 0.1mmol), Molecular sieves (100mg), add 2ml of redistilled toluene, put them into a small test tube reactor, react at room temperature for 1h, and then put them into a low-temperature reaction bath to cool to -10°C. Weigh phenyldiazoacetate methyl ester (19.4mg, 0.11mmol), 2,5-dimethoxyaniline (15.3mg, 0.1mmol), the two compounds are mixed and dissolved in 2ml of redistilled toluene, and by peristaltic Inject the pump into the reaction system for 1 hour. After the injection, continue to stir at -10°C for 1 hour, then filter, and the filtrate is rotary evaporated at 40°C to remove the solvent, and then pass column chromatography (petroleum ether: ethyl acetate = 1:30~1: 10) Isolate the pure product A of the α,β-diaminic acid derivative with the opti...
Embodiment 2
[0047]
[0048] Weigh 2,5-dimethoxyaniline (15.3mg, 0.1mmol), p-cymene dichloride ruthenium dimer (1.2mg, 0.()()lmmol), (S)-3,3 '-Triphenylsilyl substituted BINOL phosphoric acid (4.3mg, 0.005mmmol), p-chlorobenzaldehyde (14.1mg, 0.1mmol), Molecular sieves (100mg), add 2ml of redistilled toluene, put them into a small test tube reactor, react at room temperature for 1h, and then put them into a low-temperature reaction bath to cool to -10°C. Weigh phenyldiazoacetate methyl ester (19.4mg, 0.11mmol), 2,5-dimethoxyaniline (15.3mg, 0.1mmol), the two compounds are mixed and dissolved in 2ml of redistilled toluene, and by peristaltic Inject the pump into the reaction system for 1 hour. After the injection, continue to stir at -10°C for 1 hour, then filter, and the filtrate is rotary evaporated at 40°C to remove the solvent, and then pass column chromatography (petroleum ether: ethyl acetate = 1:30~1: 10) Isolate the pure product B of the α,β-diaminic acid derivative with the op...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com