Pharmaceutical compositions of anisomelic acid and the use thereof
A technology of Fangfeng oxalic acid and its composition, applied in the field of Fangfeng oxalic acid and its composition in antiviral cancer treatment, and the pharmaceutical composition of Fangfeng oxalic acid, which can solve problems such as genome instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
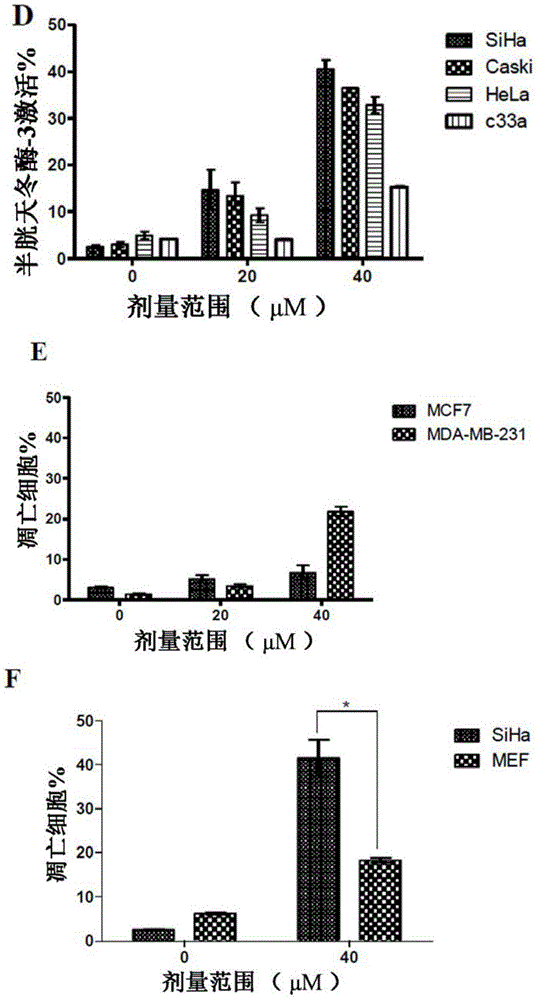
[0066] Experimental results presented below show that AA depletes viral oncoproteins E6 / E7 and inhibitor of apoptosis protein 2 (cIAP2), and thus, involves apoptosis in SiHa HPV16 positive cervical cancer cells. AA also activates the p53-p21 pathway leading to G2 / M cell cycle arrest.
[0067] The results showed that AA emulsion was more effective in inducing apoptosis at an earlier time point than AA alone. AA emulsions also effectively inhibited the growth of tumors in the CAM model.
[0068] Mammalian models are often used for preclinical evaluation of new drug delivery systems (DDS). However, valid mammalian models are expensive, time-consuming, and not easy to establish and evaluate. Furthermore, they often involve administrative burdens, both ethical and legal. Therefore, we used chorioallantoic membrane (CAM) as a surrogate for evaluating mammalian models of DDS. Study the characteristics of the CAM, embryonic anatomy, and blood to assess drug carrier properties such...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com