Pharmaceutical preparation for treatment of osteoporosis and preparation method thereof
A pharmaceutical preparation and osteoporosis technology, applied in the field of pharmaceutical preparations, can solve the problems of upper gastrointestinal mucosal irritation and not improve patients' clinical compliance with drugs, so as to promote drug dissolution, improve bioavailability, and prevent esophagus adhesion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
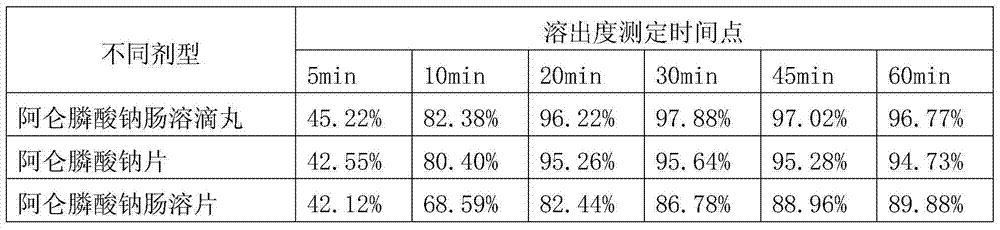
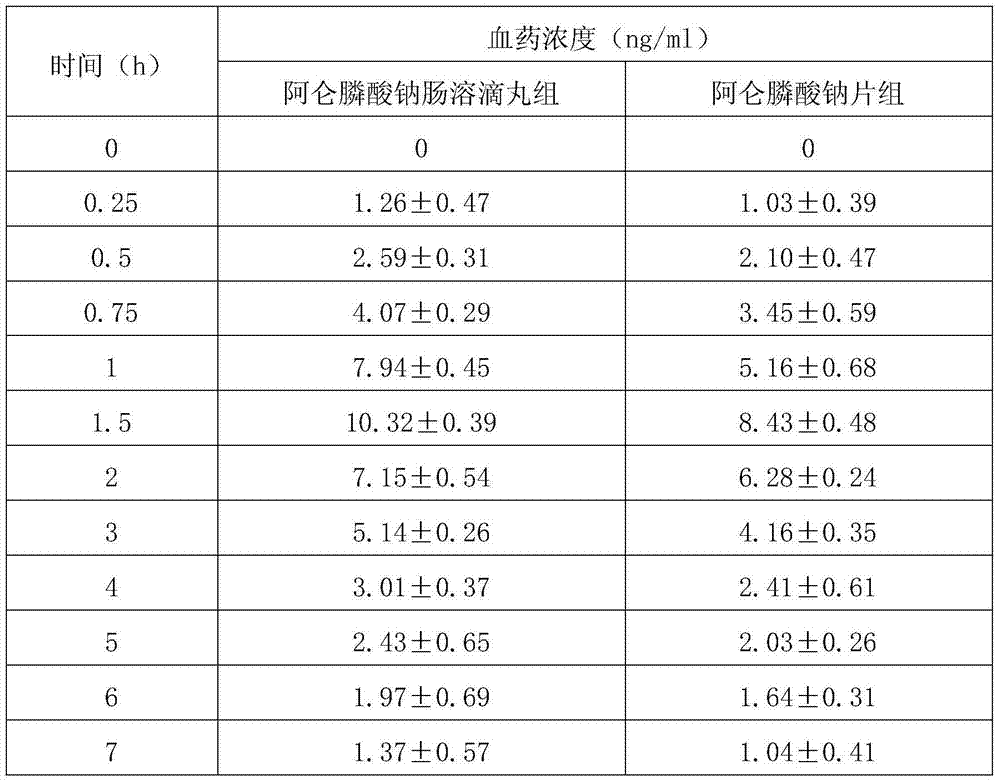
[0072] A preparation method for a pharmaceutical preparation (alendronate sodium enteric-coated dripping pill preparation) for treating osteoporosis, comprising the following steps:
[0073] 1) Take the following materials: 1kg of sodium alendronate, 1kg of water-soluble matrix, and 0.1kg of enteric coating;
[0074] 2) Mix alendronate sodium that has been crushed through a 40-mesh sieve with a water-soluble base according to the ratio described in step 1) to obtain a mixture;
[0075] 3) Melt the mixture obtained in step 2) at 60°C and keep stirring to obtain a uniform mixture;
[0076] 4) Transfer the mixed liquid obtained in step 3) to the liquid storage tank of the dripping pill machine for dripping. The dripping conditions are: the temperature of the mixed liquid is 65°C, the stirring speed of the storage tank is 30 rpm, the diameter of the dripper is 2mm, and the dripper The temperature is 60°C, the pressure of the storage tank is 0.02Mpa, the drop distance is 1cm, the ...
Embodiment 2
[0083] A preparation method for a pharmaceutical preparation (alendronate sodium enteric-coated dripping pill preparation) for treating osteoporosis, comprising the following steps:
[0084] 1) Take the following materials: sodium alendronate 1kg, water-soluble base 4kg, enteric coating 0.7kg;
[0085] 2) Mix alendronate sodium that has been crushed through an 800-mesh sieve with a water-soluble base according to the ratio described in step 1) to obtain a mixture;
[0086] 3) Melt the mixture obtained in step 2) at 90°C and keep stirring to obtain a uniform mixture;
[0087] 4) Transfer the mixed liquid obtained in step 3) to the liquid storage tank of the dripping pill machine for dripping. The dripping conditions are: the temperature of the mixed liquid is 90°C, the stirring speed of the storage tank is 90 rpm, the diameter of the dripper is 6mm, and the dripper The temperature is 85°C, the pressure of the storage tank is 0.2Mpa, the drop distance is 5cm, the temperature of...
Embodiment 3
[0094] A preparation method for a pharmaceutical preparation (alendronate sodium enteric-coated dripping pill preparation) for treating osteoporosis, comprising the following steps:
[0095] 1) Take the following materials; sodium alendronate 1kg, water-soluble matrix 2.5kg, enteric coating 0.5kg;
[0096] 2) Mix alendronate sodium that has been crushed through a 300-mesh sieve with a water-soluble base according to the ratio described in step 1) to obtain a mixture;
[0097] 3) Melt the mixture obtained in step 2) at 80°C and keep stirring to obtain a uniform mixture;
[0098] 4) Transfer the mixed liquid obtained in step 3) to the liquid storage tank of the dripping pill machine for dripping. The dripping conditions are: the temperature of the mixed liquid is 80°C, the stirring speed of the storage tank is 65 rpm, and the diameter of the dripper is 4.5mm. The head temperature is 75°C, the pressure of the storage tank is 0.06Mpa, the drop distance is 3cm, the temperature of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com