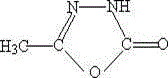
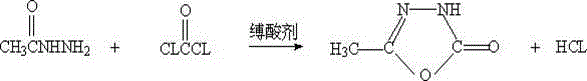
Method for synthesizing a pymetrozine intermediate (oxadiazole ketone) by utilizing carbonate ester
A technology for the production of oxadiazolone and oxadiazolone is applied in the field of carbonate synthesis of pymetrozine intermediate oxadiazolone, which can solve the problems of mass casualties, exhaust gas degradation failure, highly toxic phosgene escape and the like, To achieve the effect of mild and easy control of reaction conditions, reduction of major safety hazards, and safe and reliable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In the 1000mL acetylhydrazide reaction flask, install a stirrer, a thermometer, a constant pressure dropping funnel, a condenser and a receiving flask. Add 220-230g of 100% hydrazine hydrate, start stirring, freeze and cool down to 5-15°C, start to slowly and evenly add 400g of ethyl acetate dropwise, control the reaction temperature at 5-30°C, add the time for about 6 hours, and finish the dropping Heat preservation reaction for 2 hours, then slowly raise the temperature to 80-85°C and reflux, heat preservation reaction for 6-8 hours. Until no material is distilled out, the temperature of the kettle at the end of the decompression is 120°C (vacuum-0.098MPa), and the residual ethyl acetate and ethanol are detected to be ≤0.5% by sampling. Cool down to 80°C, transfer the acetylhydrazide into a beaker while it is still hot, and weigh it. The yield of acetylhydrazide is 95.3%, and the GC content is 99.0%.
[0052] In the oxadiazolone 500ml reaction bottle, install agitato...
Embodiment 2
[0054] Add 220-230kg of 100% hydrazine hydrate to a 1000L acetylhydrazine synthesis kettle, start stirring, freeze and cool down to 5-15°C, start to slowly and evenly add 400kg±10kg of ethyl acetate dropwise, control the reaction temperature at 5-30°C, drop The adding time is about 5-6 hours, the dropwise addition is completed and the heat preservation reaction is 2 hours, and then the temperature is slowly raised to 80-85°C for reflux, and the heat preservation reaction is 5 to 8 hours. Until no material distills out, the temperature of the kettle at the end of decompression is 130°C (pressure -0.098MPa). After the sampling test is qualified, the jacketed water is cooled to 70-80°C, weighed and filled into barrels or transferred to the oxadiazolone synthesis kettle , acetylhydrazide yield 95.0%, GC content 99.3%.
[0055] In the oxadiazolone 500ml reaction bottle, install stirrer, thermometer, condenser and rectifying column, and instrument all will dry anhydrous. At room te...
Embodiment 3
[0057] In a 2000L acetylhydrazine synthesis kettle, add 860kg of ethyl acetate, start stirring, control the temperature at 10-55°C, slowly add 600kg of hydrazine hydrate dropwise, the dropwise addition time is 3-6 hours, and the dropwise reaction is completed for 1-2 hours. , then slowly raise the temperature to reflux, and keep the temperature for 5-10 hours. After the central control is qualified, start to remove the by-products ethanol and water under normal and reduced pressure until no material is distilled. , after the sampling test is qualified, the jacketed water is cooled to 70-80°C, weighed and filled into barrels or transferred to the oxadiazolone synthesis kettle, the yield of acetylhydrazide is 94.0%, and the GC content is 99.2%.
[0058] In the oxadiazolone 1000ml reaction bottle, install agitator, thermometer, constant pressure dropping funnel and condenser, and all instruments will be dry and anhydrous. At room temperature, add 250ml of dichloroethane as a solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com