Single functional branched polyethylene glycol containing degradable radical, preparation method and biorelevant substance of single functional branched polyethylene glycol
A technology of polyethylene glycol and functionalization, which is applied in the direction of medical preparations and pharmaceutical formulations of non-active ingredients, can solve the problems of activity reduction and disappearance, increase exposure, improve drug activity reduction, and improve drug activity. effective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] In the preparation method part of the present invention, dotted lines are used in the structural formulas of some skeleton groups to indicate that the skeletons will be directly connected to the groups shown in the structural formulas in the specified compounds.
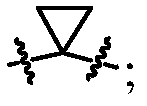
[0111] In the present invention, the ring structure is represented by a circle, and different labels are given according to the difference of the ring structure. For example,
[0112] represents any ring structure;
[0113] Represents an aliphatic ring structure without any aromatic or heteroaromatic rings, also known as aliphatic rings;
[0114] Represents an aromatic ring structure, containing at least one aromatic ring or heteroaromatic ring, also known as an aromatic ring;
[0115] Indicates the skeleton of sugars or sugar derivatives containing cyclic monosaccharide skeletons, also known as sugar rings;
[0116] Indicates a ring containing amide bonds, ester bonds, imides, acid anhydrides and...
Embodiment 1
[1090] Embodiment 1: the preparation of several key intermediates:
[1091] Preparation of Compound H1-1
[1092] In this example, the H-like compound selects the branched group is a symmetric type, and is a carbon atom branching center, L 1 =CH 2 , L 2 =CH 2 , A 1 =A 2 =CH 3 , R=OH, the protecting group PG=TBS of the hydroxyl group at the end of the symmetry axis of the small molecule initiator. The total molecular weight is designed to be about 10,000, and the molecular weight of the two branched chains is about 2*4500=9000, that is, n 1 ≈n 2 ≈102; the molecular weight of the main chain of the symmetry axis is about 1000, that is, n 3 ≈23.
[1093]
[1094] a. In an anhydrous and oxygen-free airtight reaction kettle, add tetrahydrofuran (250mL), small molecule initiator (2.532mmol) and diphenylmethyl potassium (4.0mmol) successively;
[1095] b. Add calculated amount of ethylene oxide (26.5mL), gradually heat up to a temperature of 60°C, and react for 48 ho...
Embodiment 2
[1122] Embodiment 2: Preparation of F2-1 compound
[1123]
[1124] Preparation of compound F2-1
[1125] In this example, the selected branched group of the class F compound is a symmetrical type, U is a nitrogen atom, and L 1 =CH 2 CH 2 , L 2 =CH 2 CH 2 , L 3 =CH 2 CH 2 ,A 1 =A 2 =CH 3 ,q 1 = 1, The protecting group of the terminal hydroxyl group of the main chain of the small molecule initiator is PG=acetone. The total molecular weight is designed to be about 20,000, and the molecular weight of the two branched chains is about 2*1500=3000, that is, n 1 ≈n 2 ≈34; the molecular weight of the main chain containing active functional groups is about 17000, that is, n 3 ≈396.
[1126]
[1127] a. In an anhydrous and oxygen-free airtight reaction kettle, sequentially add tetrahydrofuran (250mL), small molecule initiator 9-1 (2.532mmol) and diphenylmethyl potassium (2.0mmol);
[1128] b. Add the calculated amount of ethylene oxide (8.8mL), gradually heat up...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com