Novel triazolopyrazine derivative and use thereof
A technology of azolopyrazine and derivatives, applied in the field of pharmaceutical compositions for the prevention and treatment of abnormal proliferative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
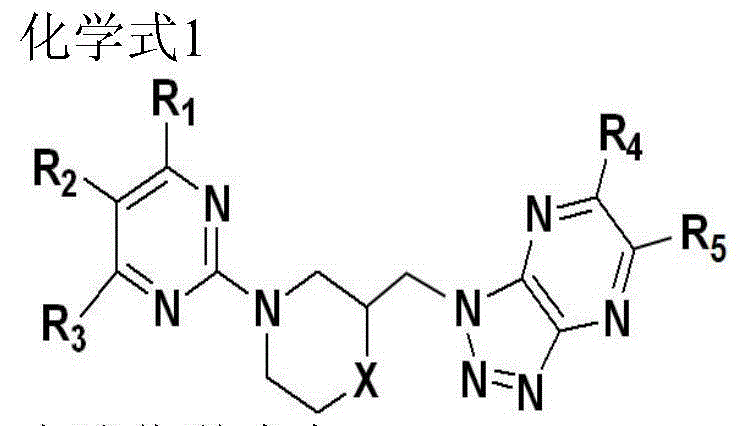
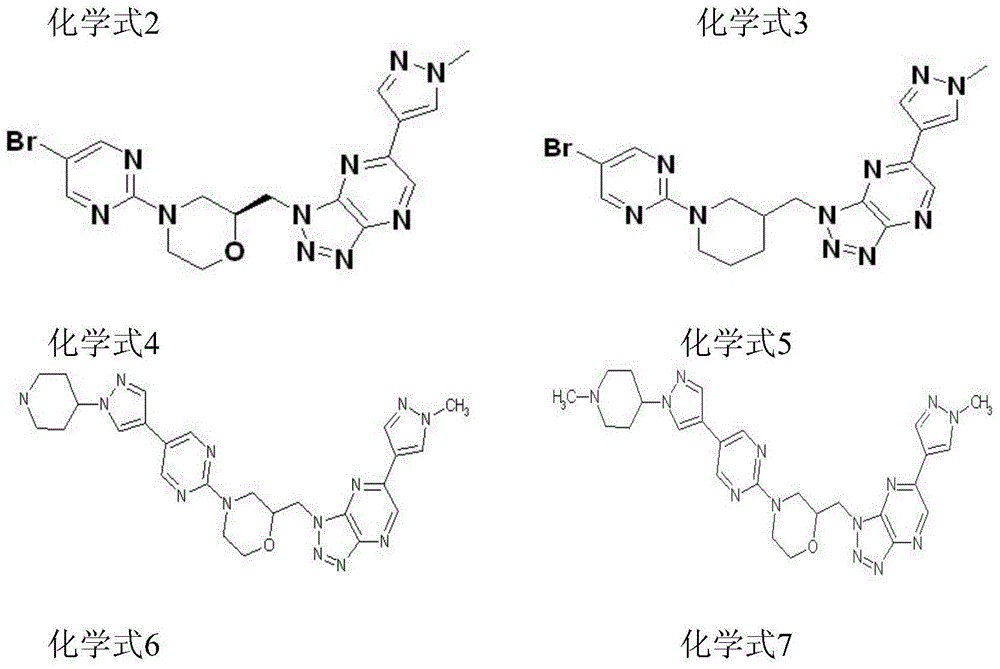
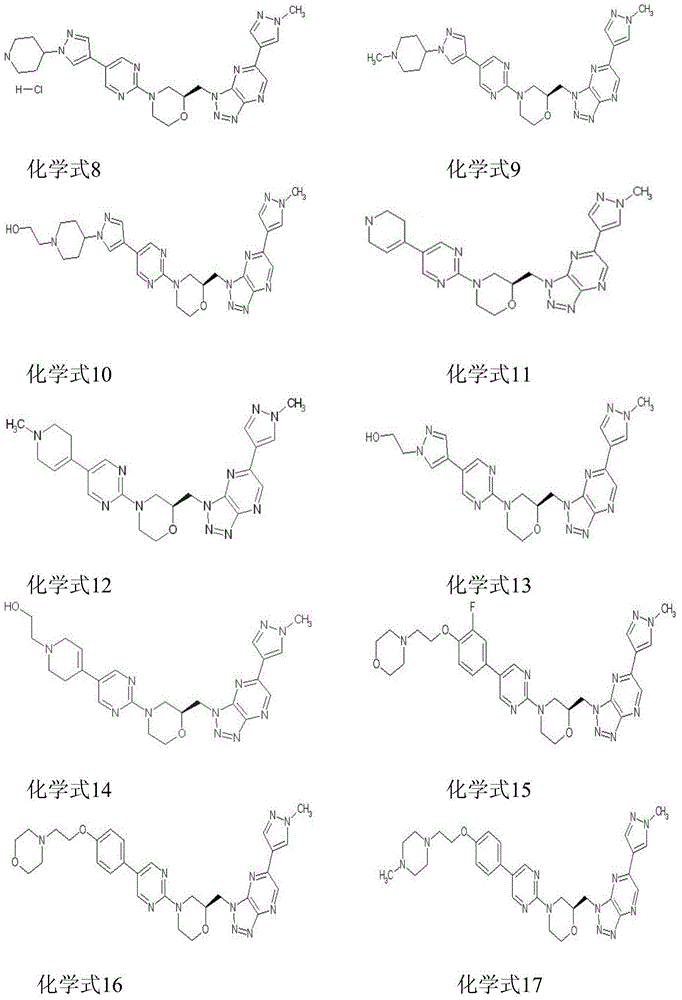
[0073] The preparation method of each compound
[0074]
[0075] (S)-(4-Benzylmorpholin-2-yl)methanol
[0076] Dissolve 38.3ml (270.22mmnol) of 2-(benzylamino)ethanol and 25g (270.22mmnol) of (R)-2-(chloromethyl)oxirane in H 2 After O / IPA (26ml+26ml), it was stirred overnight at room temperature. After that, slowly drop 130ml of Et dissolved in water over 1 hour 3 After NOH (35%), it was stirred at room temperature for 3 hours. After the reaction is over, adjust the pH to 9 with 1N HCl, and then use H 2 O and EA for extraction, drying (Na 2 SO 4 ), filtered, concentrated under reduced pressure, and the residue was purified by chromatography (EA:Hex=1:1), so as to obtain (S)-(4-benzylmorpholin-2-ylmethanol as a colorless oil (50%).
[0077] 1 H-NMR (300MHz, CDCl 3 ) δ 7.36-7.22 (m, 5H), 3.94-3.85 (m, 1H), 3.76-3.42 (m, 6H), 2.68 (d, J=11.2Hz, 2H), 2.19 (dt, J=3.1Hz , 11.2Hz, 1H), 2.00(t, J=10.9Hz, 1H)
[0078] (S)-(4-Benzylmorpholin-2-yl)methyl 4-toluenesulfonate ...
preparation example 1
[0379] Formulation Example 1: Tablet (direct compression)
[0380] After screening 5.0 mg of active ingredient, 14.1 mg of lactose, 0.8 mg of crospovidone USNF and 0.1 mg of magnesium stearate were mixed and made into tablets by compression.
preparation example 2
[0381] Formulation example 2: tablet (wet assembly)
[0382] After screening 5.0 mg of active ingredient, 16.0 mg of lactose and 4.0 mg of starch were mixed. After dissolving polysorbate 80 (0.3 mg) in purified water, an appropriate amount of the above solution was added, followed by micronization. After drying, the granules were screened and mixed with 2.7 mg of colloidal silicon dioxide and 2.0 mg of magnesium stearate. Tablets are made by compressing the granules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com