Synthesis of trifluoromethyl derivative and application of synthesis of trifluoromethyl derivative in organic electroluminescence
A technology of trifluoromethylpyridine and electroluminescent devices, applied in the synthesis and its application in OLEDs, in the field of trifluoromethyl compounds, can solve the problems of harsh reaction conditions and increased costs, and achieve the goal of improving device performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1
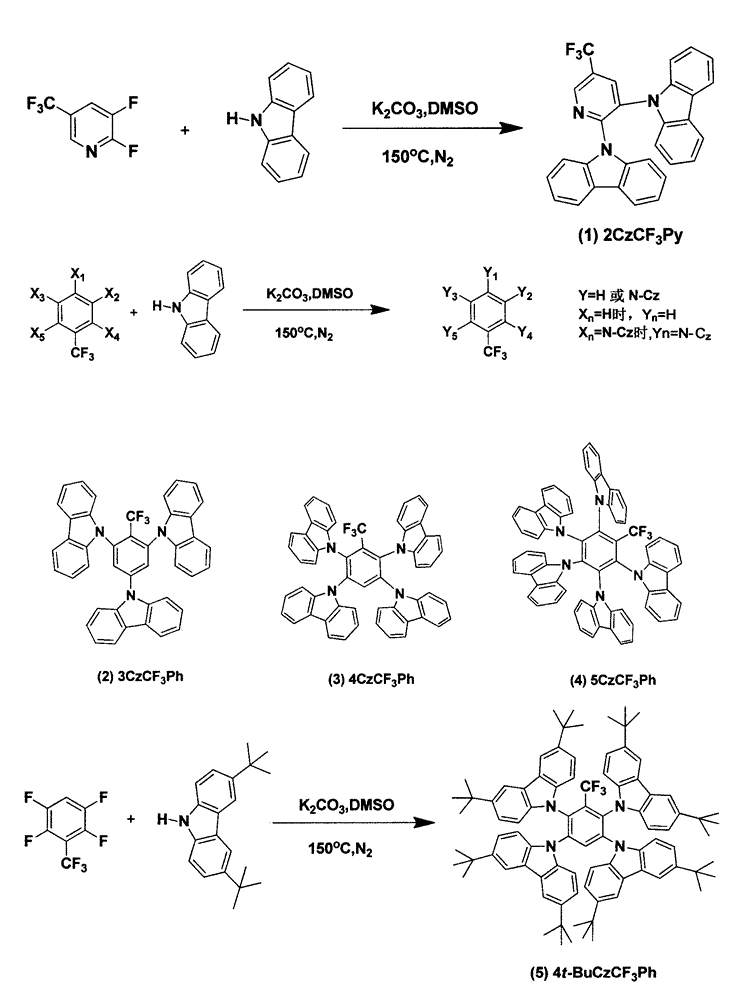
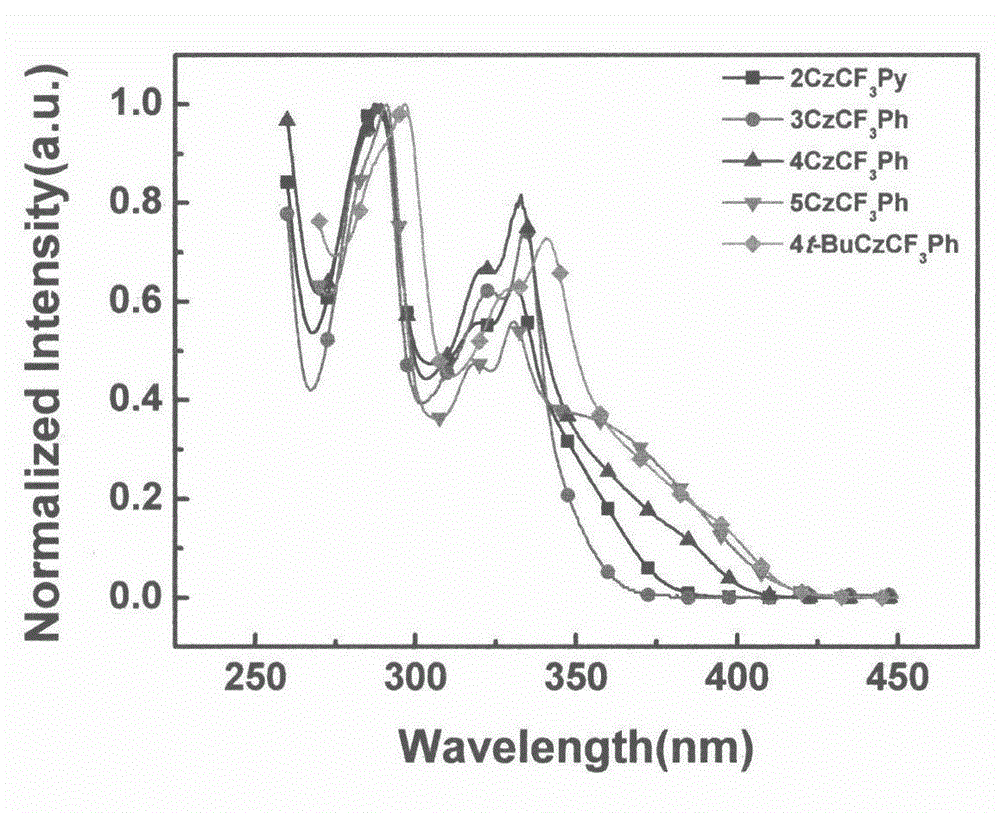
[0018] Example (1): 2CzCF 3 Synthesis of Py
[0019] 2,3-Difluorotrifluoromethylpyridine (0.25g, 1.4mmol), potassium carbonate (1.13g, 8.2mmol), carbazole (0.5g, 3.0mmol), DMSO 8ml, heated to reflux at 150°C for 12h. Cooled to room temperature and poured into 200ml of water to precipitate a large amount of solid and stirred for 0.5h, suction filtered to obtain a white solid, and purified by column chromatography to obtain a white solid with a yield of 25%. 1 H NMR (300MHz, CDCl 3 ): δppm 9.10 (s, 1H), 8.48 (1, 1H), 7.83 (m, 4H), 7.32 (s, 1H), 7.29 (s, 1H), 7.16-7.00 (m, 10H).
Embodiment (2
[0020] Example (2): 3CzCF 3 Synthesis of Ph
[0021] 2,4,6-Trifluorobenzotrifluoride (0.18g, 0.90mmol), potassium carbonate (1.0g, 7.2mmol), carbazole (0.5g, 3.0mmol), DMSO 8ml, heated to reflux at 150°C for 12h. Cool to room temperature and pour into 200ml of water to precipitate a large amount of solid, stir for 0.5h, filter with suction to obtain a white solid, and purify by column chromatography to obtain a white solid with a yield of 80%. 1 H NMR (300MHz, CDCl 3 ): δppm 8.18(d, 4H, J=3.8Hz), 8.08(d, 2H, J=3.9Hz), 7.95(s, 2H), 7.54(t, 6H, J=6.7Hz), 7.42-7.30( m, 12H).
Embodiment (3
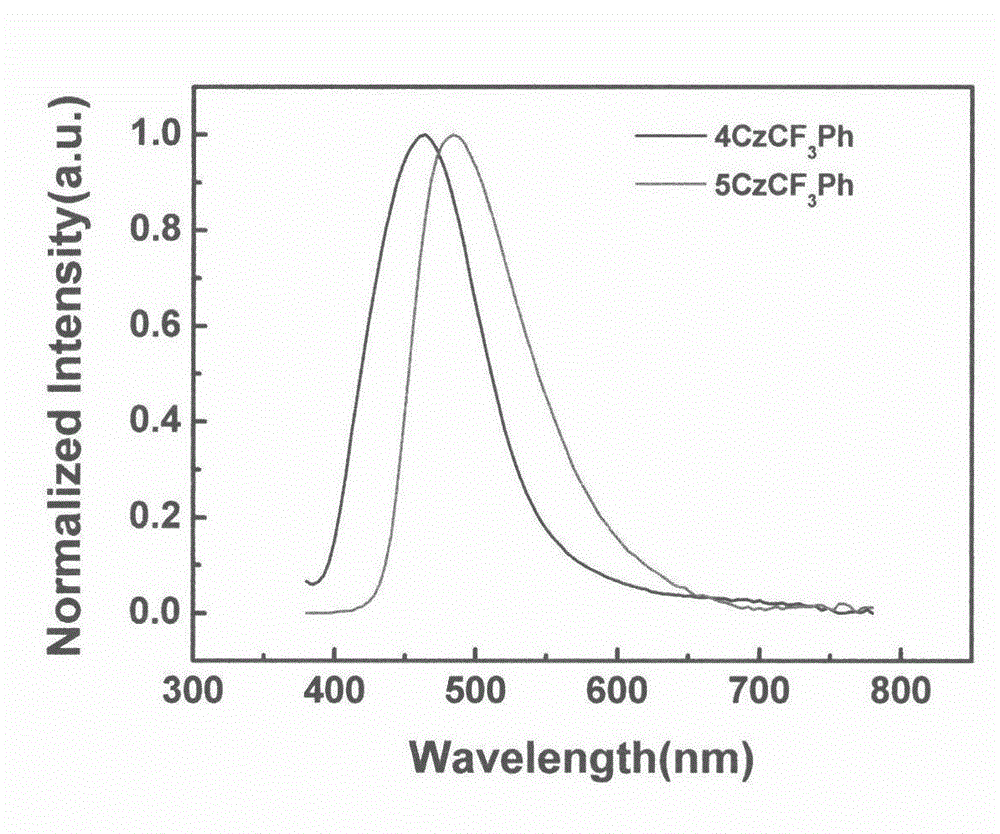
[0022] Example (3): 4CzCF 3 Synthesis of Ph
[0023] 2,3,5,6-Tetrafluorobenzotrifluoride (0.3g, 1.4mmol), potassium carbonate (1.88g, 13.6mmol), carbazole (1g, 6.0mmol), DMSO 8ml, heated to reflux at 150°C for 12h. Cool to room temperature and pour into 200ml of water to precipitate a large amount of solid, stir for 0.5h, filter with suction to obtain a white solid, and purify by column chromatography to obtain a white solid with a yield of 80%; 1 H NMR (300MHz, CDCl 3 ): δppm 8.33(s, 1H), 7.77(t, 7H, J=6.2Hz), 7.40(d, 4H, J=4.1Hz), 7.32-7.21(m, 11H), 7.17-7.06(m, 10H ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com