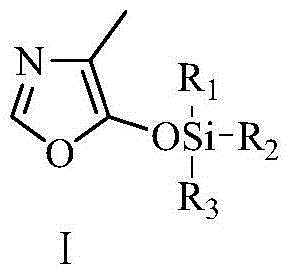
Vitamin B6 intermediate 4-methyl-5-alkylsiloxane oxazole, preparation method thereof as well as method for preparing vitamin B6
A technology for alkylsiloxy oxazole and vitamin, which is applied in the field of chemical synthesis of vitamin B6, can solve the problems of complicated preparation process, unfavorable environmental protection and high price of oxazole, and achieves high reaction selectivity, convenient source of raw materials and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: the preparation of 4-methyl-5-trimethylsiloxy oxazole
[0050] Step 1: Preparation of 4-methyltetrahydrothiazol-5-one
[0051] Add 89.0 grams (1.0 moles) of 2-aminopropionic acid, 2.0 grams of p-toluenesulfonic acid and 800 grams of Toluene, after heating up to 110°C and refluxing, add 105 grams (1.3 moles) of 37% formaldehyde solution dropwise for about 3 hours, then reflux for 5 hours for dehydration to obtain a toluene solution of 4-methyltetrahydrothiazol-5-one , used directly in step 2.
[0052] Step 2: Preparation of 4-methyl-5-trimethylsilyloxyoxazole
[0053] The toluene solution of 4-methyltetrahydrothiazol-5-one obtained in step 1 is cooled to 10° C., 440 grams (3.2 moles) of potassium carbonate are added, and 75 grams (1.06 moles) of chlorine gas is introduced between 10 and 15° C. After passing through, react at 10-15°C for 4 hours. Then, 120 g (1.1 moles) of trimethylchlorosilane was added dropwise at 10-15° C., and the reaction was carrie...
Embodiment 2
[0054] Embodiment 2: the preparation of 4-methyl-5-trimethylsiloxy oxazole
[0055] Step 1: Preparation of 4-methyltetrahydrothiazol-5-one
[0056] Add 89.0 grams (1.0 moles) of 2-aminopropionic acid, 2.0 grams of p-toluenesulfonic acid, and 400 grams of 2-Methyltetrahydrofuran, 400 grams of toluene, and 33 grams (1.1 moles) of paraformaldehyde were heated to 80-85° C. for reflux dehydration reaction for 5 hours. Cool to obtain a toluene solution of 4-methyltetrahydrothiazol-5-one, which is directly used in step 2.
[0057] Step 2: Preparation of 4-methyl-5-trimethylsilyloxyoxazole
[0058] Cool the toluene solution of 4-methyltetrahydrothiazol-5-one obtained in step 1 to 10°C, add 245 grams (3.1 moles) of pyridine, and feed 75 grams (1.06 moles) of chlorine gas between 10 and 15°C. After completion, react at 10-15°C for 4 hours. Then, 120 g (1.1 moles) of trimethylchlorosilane was added dropwise at 10-15° C., and the reaction was carried out at 10-15° C. for 3 hours. Rem...
Embodiment 3
[0059] Embodiment 3: the preparation of 4-methyl-5-trimethylsiloxy oxazole
[0060] Step 1: Preparation of 4-methyltetrahydrothiazol-5-one
[0061] Add 89.0 grams (1.0 moles) of 2-aminopropionic acid, 1.8 grams of benzenesulfonic acid and 800 grams of toluene to a 2000 mL four-necked flask equipped with stirring, a thermometer, a constant pressure dropping funnel, a reflux condenser and a water separator, and heat up After refluxing to 110° C., 105 g (1.3 moles) of 37% formaldehyde aqueous solution was added dropwise for about 3 hours, and then refluxed for dehydration for 6 hours. Cool to obtain a toluene solution of 4-methyltetrahydrothiazol-5-one, which is directly used in step 2.
[0062] Step 2: Preparation of 4-methyl-5-trimethylsilyloxyoxazole
[0063] The toluene solution of 4-methyltetrahydrothiazol-5-one obtained in step 1 is transferred to the equipment equipped with stirring, thermometer, constant pressure dropping funnel, reflux condenser and tail gas absorption...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com